Carsten von Hänisch
@haenisch-group.bsky.social
100 followers
49 following
9 posts
Working group for inorganic chemistry with a preference for the main group elements ;)
Molecular Chemistry
Coordination chemistry and
Low oxidation states
Posts
Media
Videos
Starter Packs
Reposted by Carsten von Hänisch
Fabian Dankert
@fabiandankert.bsky.social
· Aug 25

Functional Al/Cd Heterometallics─From Controlled Al(I) Transfer to Nucleophilic Transfer of Cadmium Ions
Low-valent cadmium compounds have remained largely unexplored as electron reservoirs, with no precedent for their use in reduction or bond activation chemistry. Here, we address this gap by integratin...
pubs.acs.org
Reposted by Carsten von Hänisch
Schneider Lab
@schneider-lab.bsky.social
· Jan 29
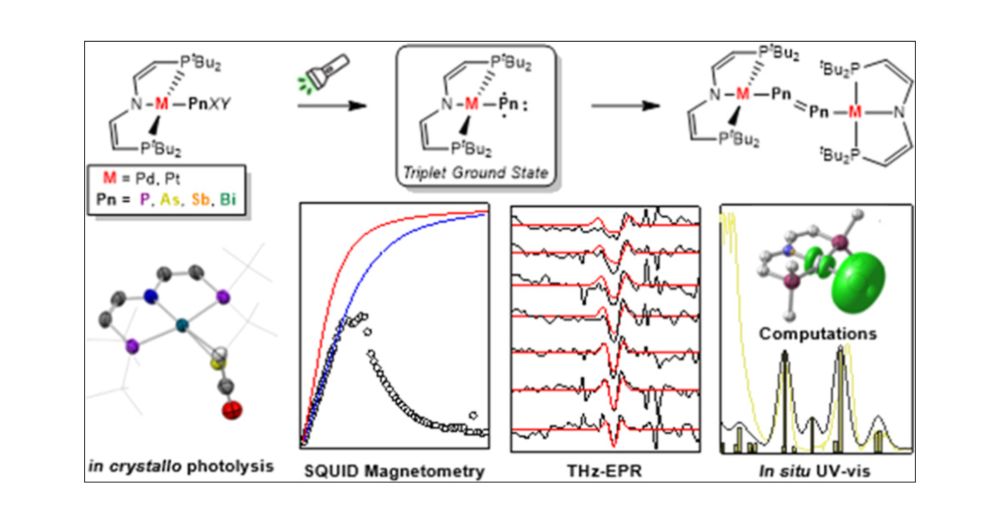
Transient Triplet Metallopnictinidenes M–Pn (M = PdII, PtII; Pn = P, As, Sb): Characterization and Dimerization
Nitrenes (R–N) have been subject to a large body of experimental and theoretical studies. The fundamental reactivity of this important class of transient intermediates has been attributed to their electronic structures, particularly the accessibility of triplet vs singlet states. In contrast, electronic structure trends along the heavier pnictinidene analogues (R–Pn; Pn = P–Bi) are much less systematically explored. We here report the synthesis of a series of metallodipnictenes, {M–Pn═Pn–M} (M = PdII, PtII; Pn = P, As, Sb, Bi) and the characterization of the transient metallopnictinidene intermediates, {M–Pn} for Pn = P, As, Sb. Structural, spectroscopic, and computational analysis revealed spin triplet ground states for the metallopnictinidenes with characteristic electronic structure trends along the series. In comparison to the nitrene, the heavier pnictinidenes exhibit lower-lying ground state SOMOs and singlet excited states, thus suggesting increased electrophilic reactivity. Furthermore, the splitting of the triplet magnetic microstates is beyond the phosphinidenes {M–P} dominated by heavy pnictogen atom induced spin–orbit coupling.
pubs.acs.org