Fabian Dankert
@fabiandankert.bsky.social
120 followers
140 following
12 posts
Group Leader @Universität Kassel pursuing research in multimetallics for bond activation and catalysis. | First generation academic. | Liebig Fellow.
Posts
Media
Videos
Starter Packs
Reposted by Fabian Dankert
Fabian Dankert
@fabiandankert.bsky.social
· Aug 27
Fabian Dankert
@fabiandankert.bsky.social
· Aug 27
Fabian Dankert
@fabiandankert.bsky.social
· Aug 25
Fabian Dankert
@fabiandankert.bsky.social
· Aug 25

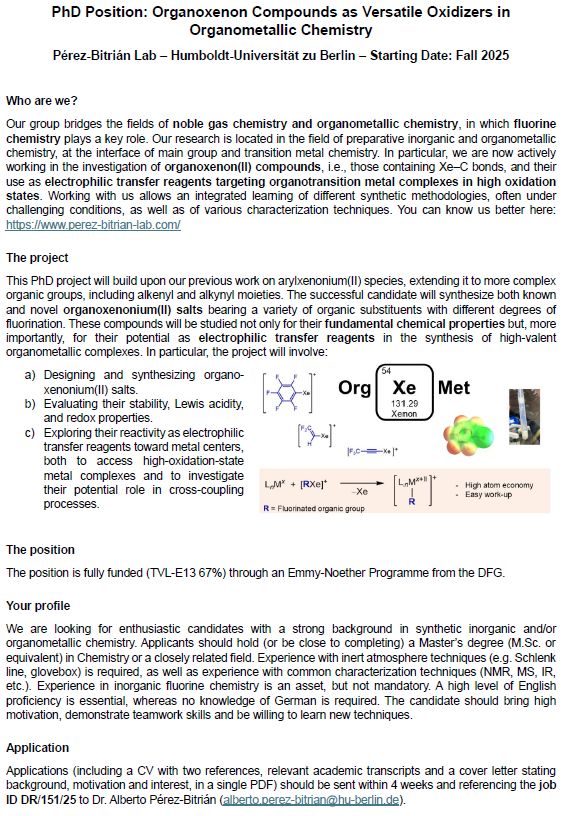
Functional Al/Cd Heterometallics─From Controlled Al(I) Transfer to Nucleophilic Transfer of Cadmium Ions
Low-valent cadmium compounds have remained largely unexplored as electron reservoirs, with no precedent for their use in reduction or bond activation chemistry. Here, we address this gap by integratin...
pubs.acs.org
Fabian Dankert
@fabiandankert.bsky.social
· Aug 14
Reposted by Fabian Dankert
Reposted by Fabian Dankert
Reposted by Fabian Dankert
Malte Fischer
@maltefischer.bsky.social
· Jul 22
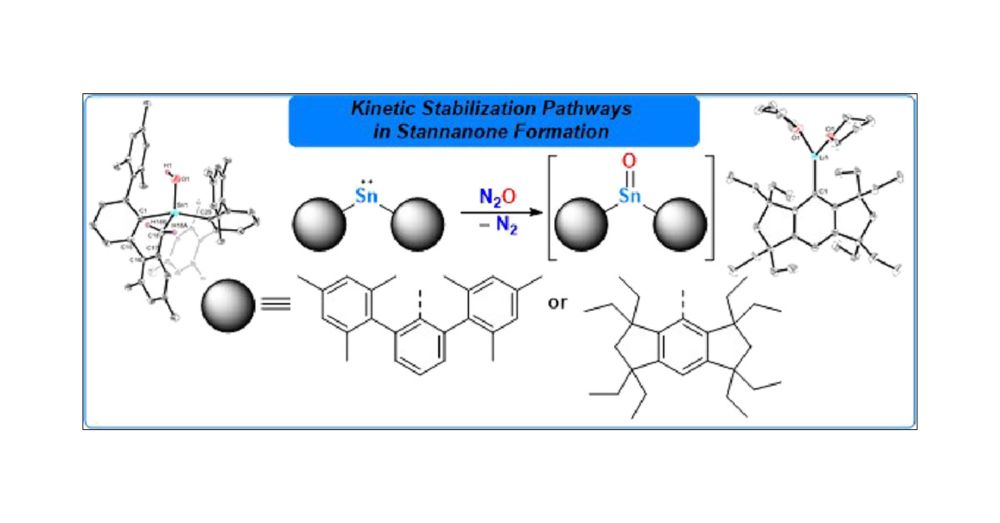
Kinetic Stabilization in Diaryl-Substituted Stannylenes: N2O Reactivity, Intramolecular C–H Activation, and Crystalline (Eind)Li(THF)2 as a Versatile Precursor in Tin Chemistry
The reactivity of the kinetically stabilized stannylene (MesTer)2Sn (1) (MesTer = –C6H3-2,6-(2,4,6-Me3-C6H2)2) toward N2O is revisited, yielding the terminal tin(IV) hydroxide 2 via formal intramolecular C(sp3)–H activation of a putative terminal stannanone intermediate. By switching to Eind ligation (Eind = 1,1,3,3,5,5,7,7-octaethyl-s-hydrindacen-4-yl) at the tin center, the synthesis and characterization of the crystalline lithium salt (Eind)Li(THF)2 (3) is reported, serving as a straightforward precursor for the clean generation of the corresponding stannylene (Eind)2Sn (4). Compound 4 can be further cleanly converted into the heteroleptic Eind/halide stannylene (Eind)SnCl (6). Both 4 and 6 serve as suitable precursors for the synthesis of the heteroleptic s-hydrindacene-/amido-substituted stannylene (Eind)Sn{N(SiMe3)2} (5).
pubs.acs.org
Fabian Dankert
@fabiandankert.bsky.social
· Jul 10
Reposted by Fabian Dankert
Universität Kassel
@unikassel.bsky.social
· Jun 12
Reposted by Fabian Dankert
Fabian Dankert
@fabiandankert.bsky.social
· Apr 26
Reposted by Fabian Dankert
Reposted by Fabian Dankert
Andryj Borys
@andryjborys.bsky.social
· Apr 12

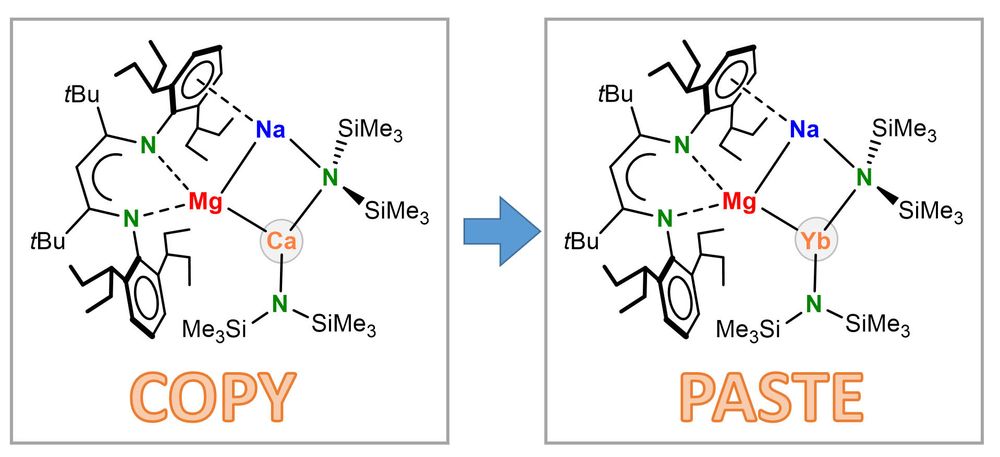
Magnesium Nickelate Complexes and Their Implications in Ni-Catalyzed Cross-Couplings of Aryl Fluorides and Aryl Ethers with Grignard Reagents
The nickel-catalyzed Kumada–Tamao–Corriu cross-coupling reaction is widely used to form C–C bonds and receives continued interest due to the unique ability of nickel to activate challenging organic electrophiles containing C–F and C–O bonds. Recent studies on the nickel-catalyzed cross-coupling of Ar–F and Ar–OMe electrophiles with organolithium nucleophiles have unveiled the key involvement of highly reactive anionic nickelates, in which Li and Ni cooperatively promote the activation of these substrates. However, the possible formation of related heterobimetallic intermediates when employing organomagnesium nucleophiles as cross-coupling partners still remains widely underexplored. Filling this gap in the knowledge, we use air-stable Ni-tris(olefin), Ni(4-Me-stb)3 (where stb = stilbene), as a Ni(0) precursor to systematically investigate its reactivity toward several organomagnesium and organolithium reagents, which has allowed for the structural and spectroscopic characterization of a new family of nickelate complexes. Their possible implications and the influence of their constitution and speciation on the outcome of Kumada–Tamao–Corriu cross-couplings are also investigated through a series of stoichiometric and catalytic reactions, which have uncovered a dramatic solvent effect, hinting at the formation of contacted ion pair species as key to maximizing Mg (or Li)/Ni(0) cooperativity.
pubs.acs.org
Reposted by Fabian Dankert
Reposted by Fabian Dankert
Neal Mankad
@metallacycle.bsky.social
· Apr 3
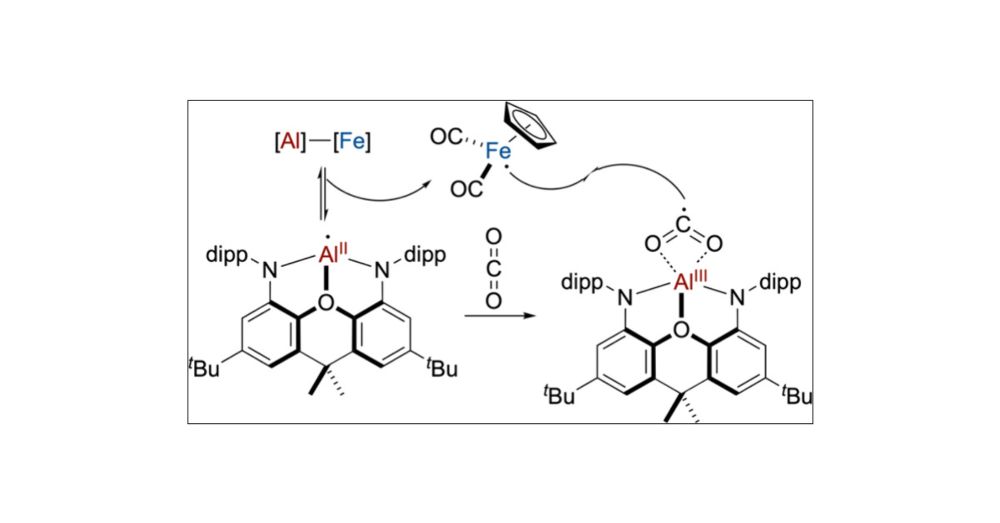
Cooperative Heterobimetallic CO2 Activation Involving a Mononuclear Aluminum(II) Intermediate
Molecular chemistry of aluminum most commonly involves AlIII ions due to their noble gas electronic configurations. In contrast, the chemistry of AlII ions is underexplored and may contain undiscovere...
doi.org
Fabian Dankert
@fabiandankert.bsky.social
· Mar 15