@coburgerpeter.bsky.social
78 followers
86 following
3 posts
www.coburger-lab.de
We are a research group @ TUM, focussing on the reactivity of biradicaloid complexes.
Posts
Media
Videos
Starter Packs
Reposted
Reposted
Reposted
Reposted
Reposted
Connie Lu
@cluchem.bsky.social
· Aug 8
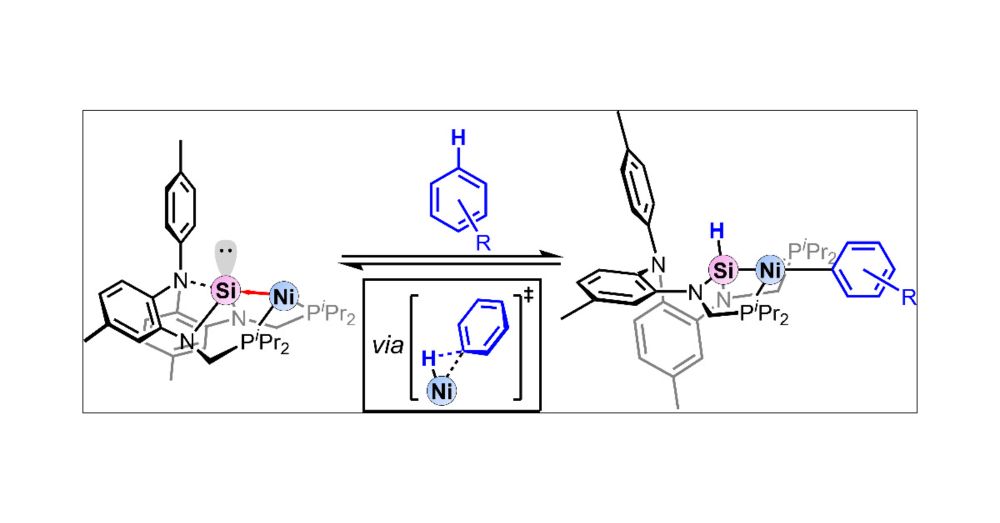
Reversible C–H Bond Activation of Unactivated Arenes by a Nickel-Silylene Complex
A nickel-silylene complex is shown to reversibly activate benzene via C–H bond activation at ambient temperature. The benzene C–H bond formally adds across the Ni–Si core to form a nickel-silyl comple...
pubs.acs.org
Reposted
Malte Fischer
@maltefischer.bsky.social
· Jul 22
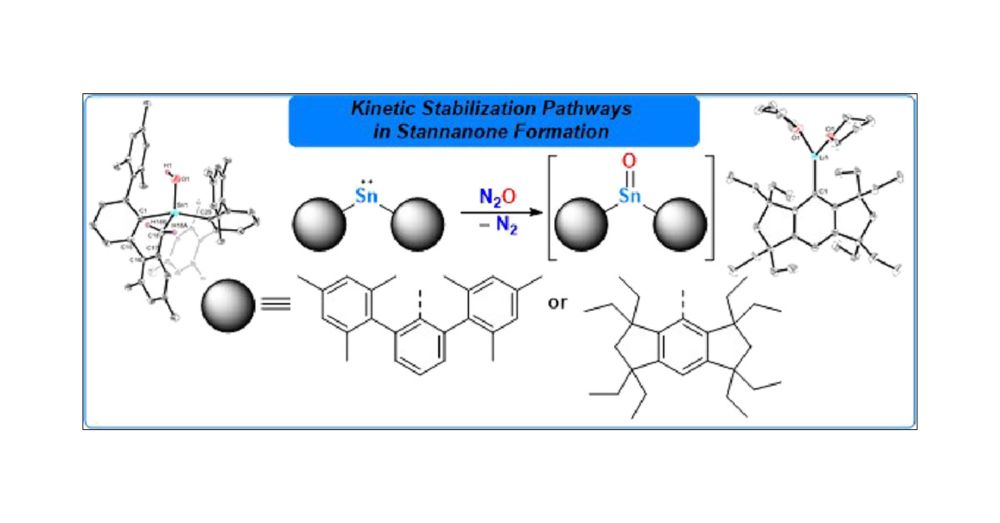
Kinetic Stabilization in Diaryl-Substituted Stannylenes: N2O Reactivity, Intramolecular C–H Activation, and Crystalline (Eind)Li(THF)2 as a Versatile Precursor in Tin Chemistry
The reactivity of the kinetically stabilized stannylene (MesTer)2Sn (1) (MesTer = –C6H3-2,6-(2,4,6-Me3-C6H2)2) toward N2O is revisited, yielding the terminal tin(IV) hydroxide 2 via formal intramolecular C(sp3)–H activation of a putative terminal stannanone intermediate. By switching to Eind ligation (Eind = 1,1,3,3,5,5,7,7-octaethyl-s-hydrindacen-4-yl) at the tin center, the synthesis and characterization of the crystalline lithium salt (Eind)Li(THF)2 (3) is reported, serving as a straightforward precursor for the clean generation of the corresponding stannylene (Eind)2Sn (4). Compound 4 can be further cleanly converted into the heteroleptic Eind/halide stannylene (Eind)SnCl (6). Both 4 and 6 serve as suitable precursors for the synthesis of the heteroleptic s-hydrindacene-/amido-substituted stannylene (Eind)Sn{N(SiMe3)2} (5).
pubs.acs.org
Reposted
Reposted
Reposted
Dalton Transactions
@daltontrans.rsc.org
· May 26
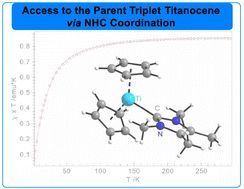
Isolation of the parent triplet titanocene via NHC stabilisation
We present the synthesis and characterization of the parent isolable monomeric triplet titanocene complex, stabilized by the N-heterocyclic carbene (NHC) IMe4. Investigated by SQUID magnetometry and…
pubs.rsc.org
Reposted
Reposted
Reposted
Reposted
RiedelLab
@riedellab.bsky.social
· May 8
Rethinking Chlorine: Essential Chemical or Replaceable Risk?
Chlorine is essential for the production of plastics and pharmaceuticals but poses significant safety and environmental risks. Herein, processes are presented that substitute chlorine or reduce its d...
chemistry-europe.onlinelibrary.wiley.com
Reposted