Malte Fischer
@maltefischer.bsky.social
230 followers
250 following
14 posts
"Fishing" around in early transition metal and main group chemistry // TT Ass. Prof. @ Georg-August-Universität Göttingen // Liebig fellow // https://www.uni-goettingen.de/de/674212.html
Posts
Media
Videos
Starter Packs
Pinned
Malte Fischer
@maltefischer.bsky.social
· Jul 22
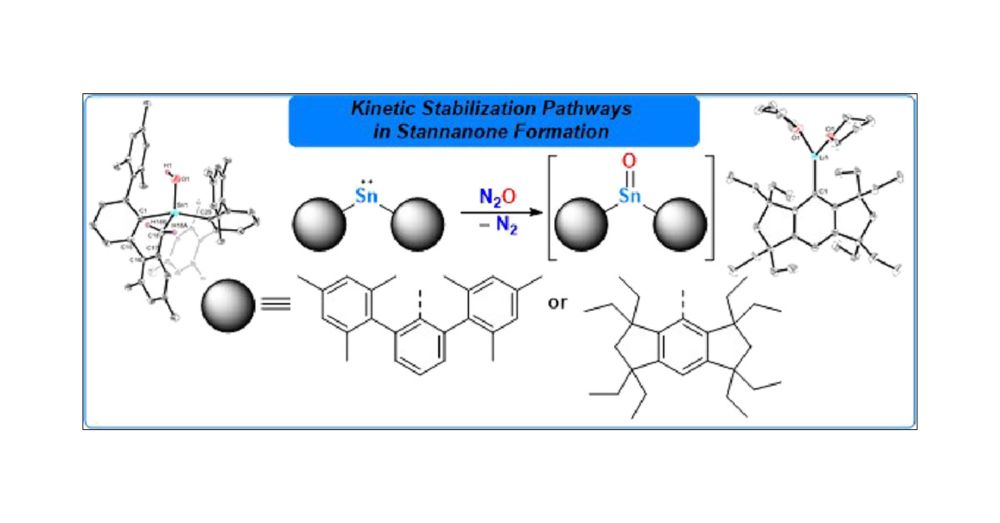
Kinetic Stabilization in Diaryl-Substituted Stannylenes: N2O Reactivity, Intramolecular C–H Activation, and Crystalline (Eind)Li(THF)2 as a Versatile Precursor in Tin Chemistry
The reactivity of the kinetically stabilized stannylene (MesTer)2Sn (1) (MesTer = –C6H3-2,6-(2,4,6-Me3-C6H2)2) toward N2O is revisited, yielding the terminal tin(IV) hydroxide 2 via formal intramolecular C(sp3)–H activation of a putative terminal stannanone intermediate. By switching to Eind ligation (Eind = 1,1,3,3,5,5,7,7-octaethyl-s-hydrindacen-4-yl) at the tin center, the synthesis and characterization of the crystalline lithium salt (Eind)Li(THF)2 (3) is reported, serving as a straightforward precursor for the clean generation of the corresponding stannylene (Eind)2Sn (4). Compound 4 can be further cleanly converted into the heteroleptic Eind/halide stannylene (Eind)SnCl (6). Both 4 and 6 serve as suitable precursors for the synthesis of the heteroleptic s-hydrindacene-/amido-substituted stannylene (Eind)Sn{N(SiMe3)2} (5).
pubs.acs.org
Reposted by Malte Fischer
Reposted by Malte Fischer
Fabian Dankert
@fabiandankert.bsky.social
· Aug 25

Functional Al/Cd Heterometallics─From Controlled Al(I) Transfer to Nucleophilic Transfer of Cadmium Ions
Low-valent cadmium compounds have remained largely unexplored as electron reservoirs, with no precedent for their use in reduction or bond activation chemistry. Here, we address this gap by integratin...
pubs.acs.org
Reposted by Malte Fischer
Reposted by Malte Fischer
Malte Fischer
@maltefischer.bsky.social
· Jul 22

Kinetic Stabilization in Diaryl-Substituted Stannylenes: N2O Reactivity, Intramolecular C–H Activation, and Crystalline (Eind)Li(THF)2 as a Versatile Precursor in Tin Chemistry
The reactivity of the kinetically stabilized stannylene (MesTer)2Sn (1) (MesTer = –C6H3-2,6-(2,4,6-Me3-C6H2)2) toward N2O is revisited, yielding the terminal tin(IV) hydroxide 2 via formal intramolecular C(sp3)–H activation of a putative terminal stannanone intermediate. By switching to Eind ligation (Eind = 1,1,3,3,5,5,7,7-octaethyl-s-hydrindacen-4-yl) at the tin center, the synthesis and characterization of the crystalline lithium salt (Eind)Li(THF)2 (3) is reported, serving as a straightforward precursor for the clean generation of the corresponding stannylene (Eind)2Sn (4). Compound 4 can be further cleanly converted into the heteroleptic Eind/halide stannylene (Eind)SnCl (6). Both 4 and 6 serve as suitable precursors for the synthesis of the heteroleptic s-hydrindacene-/amido-substituted stannylene (Eind)Sn{N(SiMe3)2} (5).
pubs.acs.org
Reposted by Malte Fischer
Reposted by Malte Fischer
Reposted by Malte Fischer
Reposted by Malte Fischer
Dalton Transactions
@daltontrans.rsc.org
· May 26
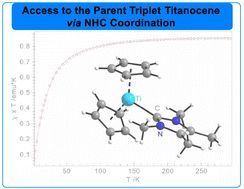
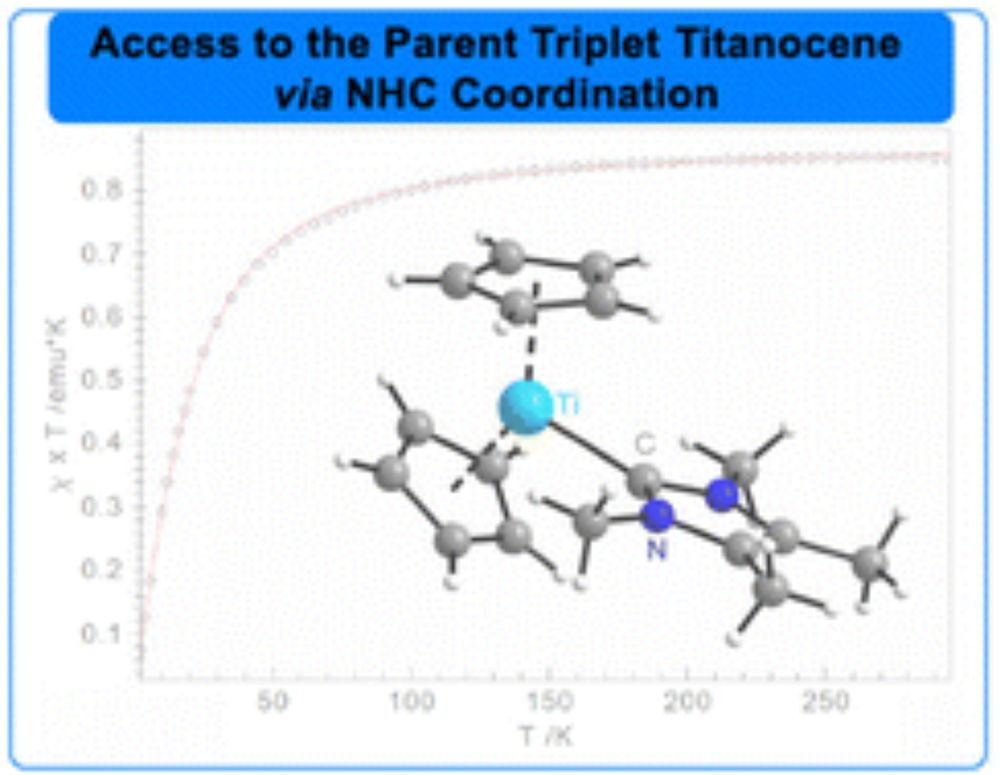
Isolation of the parent triplet titanocene via NHC stabilisation
We present the synthesis and characterization of the parent isolable monomeric triplet titanocene complex, stabilized by the N-heterocyclic carbene (NHC) IMe4. Investigated by SQUID magnetometry and…
pubs.rsc.org
Reposted by Malte Fischer
Reposted by Malte Fischer
Malte Fischer
@maltefischer.bsky.social
· Mar 21
Malte Fischer
@maltefischer.bsky.social
· Mar 21
Malte Fischer
@maltefischer.bsky.social
· Mar 19
Malte Fischer
@maltefischer.bsky.social
· Mar 19
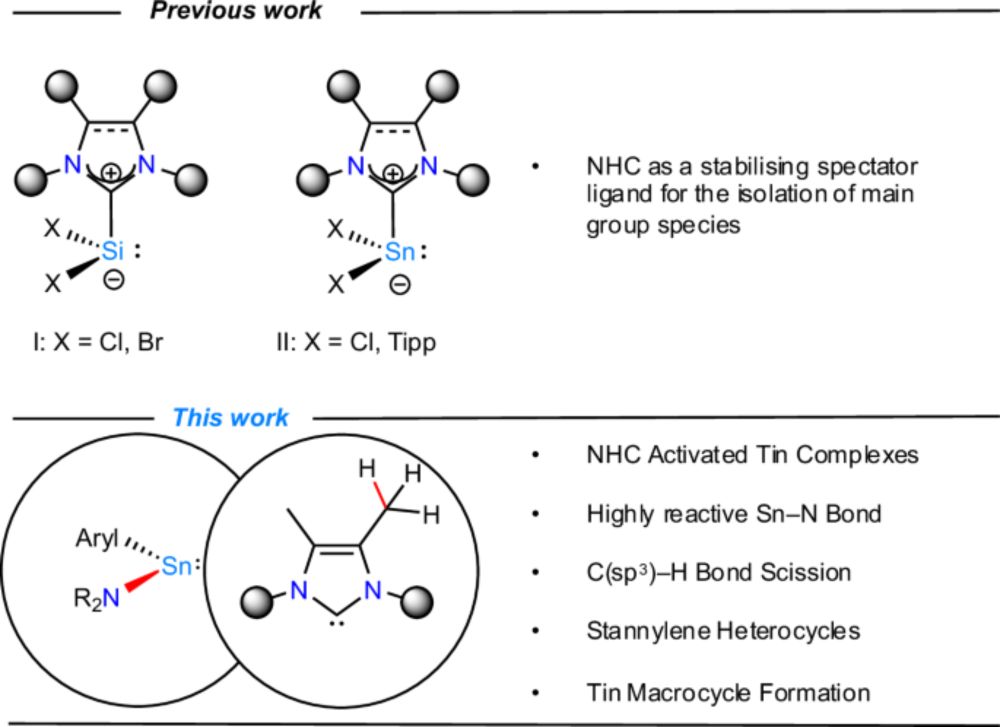
Carbene-activated stannylenes to access selective C(sp3)–H bond scission at the steric limit - Nature Communications
The ubiquity of N-heterocyclic carbenes (NHCs) in chemical research typically arises from their potent stabilizing capabilities and role as innocent spectators to stabilize otherwise non-bottleable co...
www.nature.com
Malte Fischer
@maltefischer.bsky.social
· Mar 13
Reposted by Malte Fischer
Josef Boronski
@josefboronski.bsky.social
· Mar 11
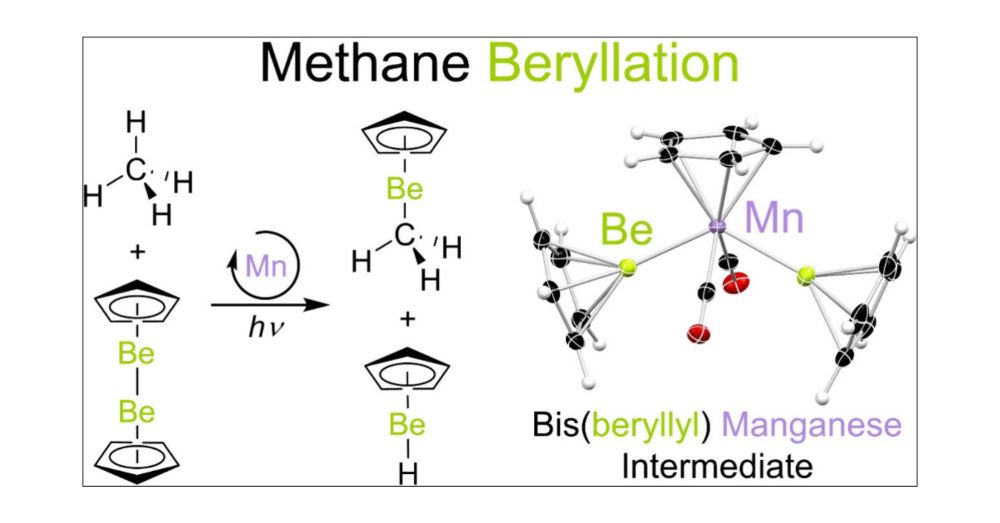
Methane Beryllation Catalyzed by a Base Metal Complex
The homogeneous catalytic functionalization of methane is extremely challenging due to the relative nonpolarity and high C–H bond strength of this hydrocarbon. Here, using catalytic quantities (10 mol...
pubs.acs.org
Reposted by Malte Fischer
Reposted by Malte Fischer