Harder Research Group
@harder-research.bsky.social
430 followers
200 following
35 posts
Organometallic Main Group Metal Chemistry, mainly s- and p-block but nowadays also some d- and f-block. @FAU Erlangen
Posts
Media
Videos
Starter Packs
Reposted by Harder Research Group
Reposted by Harder Research Group
Josef Boronski
@josefboronski.bsky.social
· Aug 11

Alkaline earth metals: heterometallic bonding
The alkaline earth metals are notorious for their tendency to form ionic compounds of their dications. In recent years, however, chemists have found ways to kinetically skirt around the edges of these...
pubs.rsc.org
Connie Lu
@cluchem.bsky.social
· Aug 8
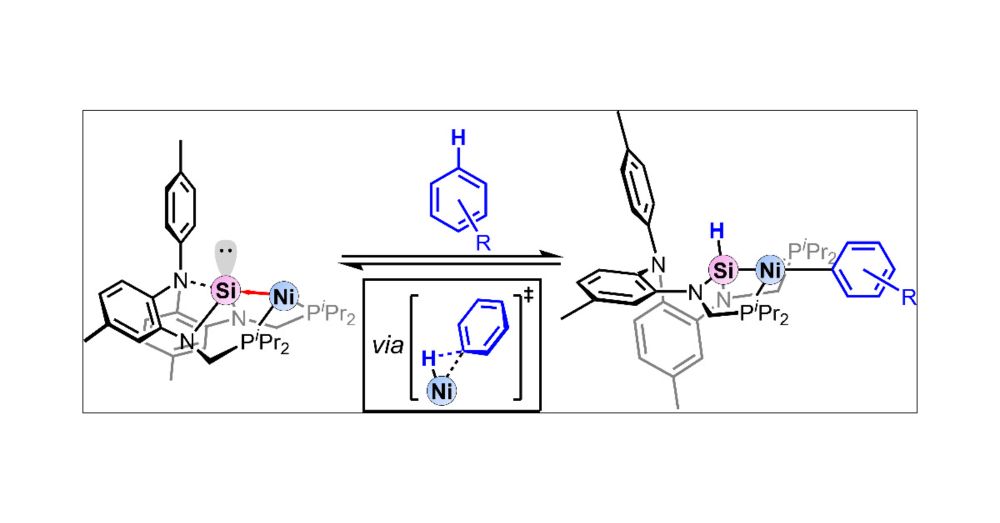
Reversible C–H Bond Activation of Unactivated Arenes by a Nickel-Silylene Complex
A nickel-silylene complex is shown to reversibly activate benzene via C–H bond activation at ambient temperature. The benzene C–H bond formally adds across the Ni–Si core to form a nickel-silyl comple...
pubs.acs.org
Reposted by Harder Research Group
Kyle Pearce
@kylegpearce.bsky.social
· Aug 2
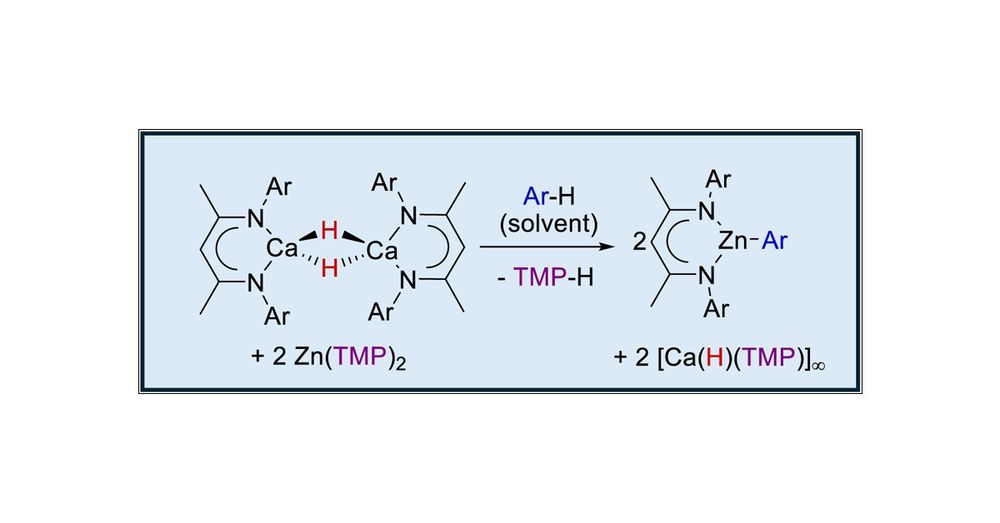
Arene Activation by Calcium Hydride/Zinc Amide Equilibration
The β-diketiminato calcium hydride, [(BDI)CaH]2 (BDI = HC{(Me)CNDipp}2, where Dipp = 2,6-i-Pr2C6H3), reacts with [Zn{N(SiMe3)2}2] and [Zn(TMP)2] (TMP = 2,2,6,6-tetramethylpiperidide) to provide labile...
pubs.acs.org
Reposted by Harder Research Group
Reposted by Harder Research Group
Dalton Transactions
@daltontrans.rsc.org
· Mar 13
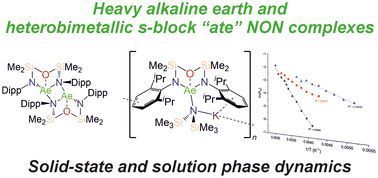
Heavier alkaline earth and heterobimetallic s-block “ate” complexes of a di(amido)siloxane ligand: solid-state structure and dynamic solution-phase behaviour
The diverse solid-state structures and solution-phase dynamics of both neutral and heterometallic s-block “ate” complexes of the heavier alkaline earth metals (Ae; Ca–Ba) supported by a chelating and…
pubs.rsc.org
Reposted by Harder Research Group
Reposted by Harder Research Group
Martyn Coles
@coles-lab.bsky.social
· Jul 21
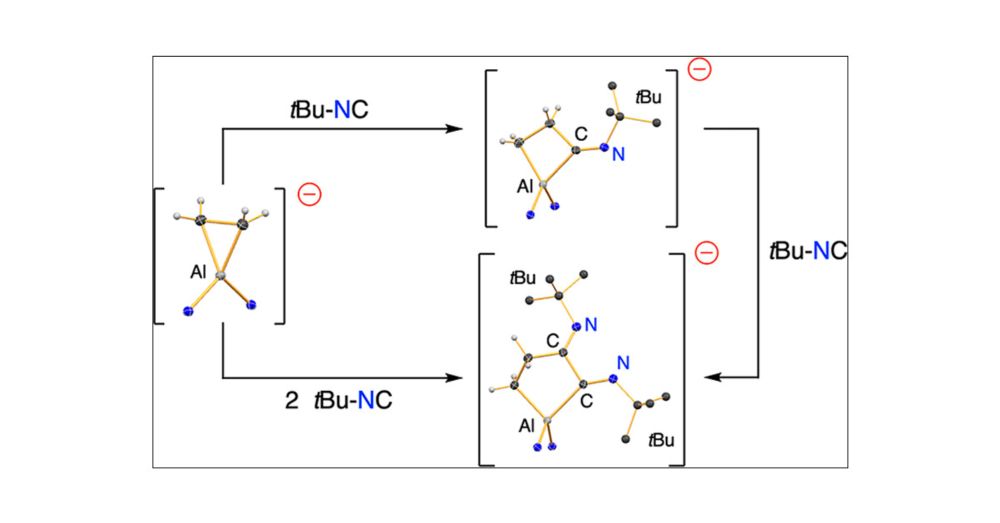
C–C Bond Formation at an Aluminacyclopropane
The reactivity of the aluminacyclopropane compound K[Al(NON)(η2-C2H4)] (NON = κN,N’-[O(SiMe2NDipp)2]2–; Dipp = 2,6-iPr2C6H3) toward isocyanides R–NC (R = Cy, Ad, tBu; Cy = cyclohexyl, Ad = 1-adamantyl...
pubs.acs.org
Reposted by Harder Research Group
Reposted by Harder Research Group
Jiaxiang Chu
@jiaxiangchu.bsky.social
· Jul 2
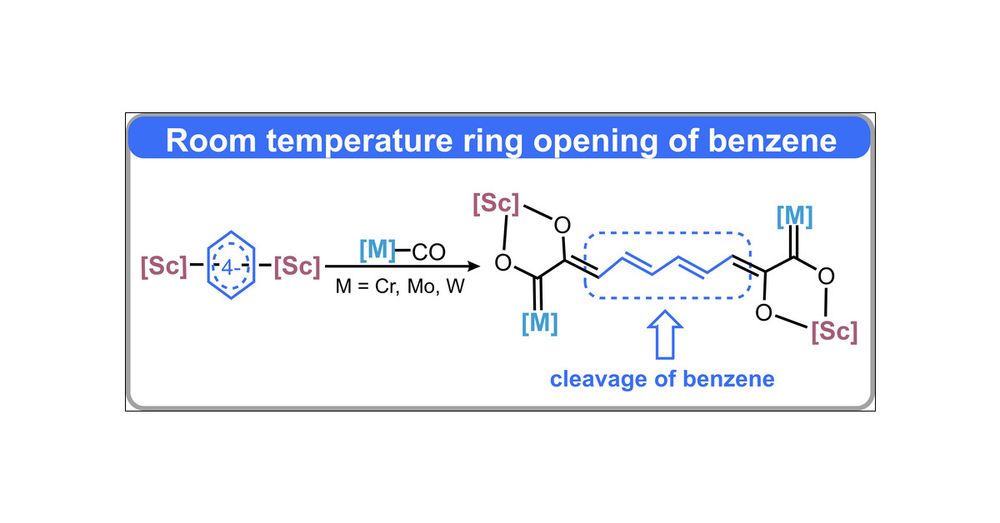
Room Temperature Ring Opening of Benzene by Four-Electron Reduction and Carbonylation
Cleaving a benzene ring under mild conditions requires disrupting its aromaticity, which is a significant challenge in modern chemistry. Although the generation of cyclic products from cleaving benzen...
pubs.acs.org
Reposted by Harder Research Group
Reposted by Harder Research Group
FAU Faculty of Sciences
@nat.fau.de
· Jun 17

Zwei ERC Advanced Grants für Prof. Dr. Maria Chekhova und Prof. Dr. Sjoerd Harder
Ein erneuter Beweis für die Forschungsstärke am Wissenschaftsstandort Erlangen-Nürnberg: Zwei Forschende der Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) sind jetzt mit jeweils einem der…
www.nat.fau.de