Josh Abbenseth
@joshabbenseth.bsky.social
210 followers
220 following
37 posts
Lecturer in Inorganic Chemistry at UoM | Organometallic/main group chemist | First generation academic | www.abbenseth-lab.com
Posts
Media
Videos
Starter Packs
Reposted by Josh Abbenseth
Reposted by Josh Abbenseth
Reposted by Josh Abbenseth
Josh Abbenseth
@joshabbenseth.bsky.social
· Aug 14
Reposted by Josh Abbenseth
Josh Abbenseth
@joshabbenseth.bsky.social
· Jul 27
Reposted by Josh Abbenseth
Reposted by Josh Abbenseth
Malte Fischer
@maltefischer.bsky.social
· Jul 22
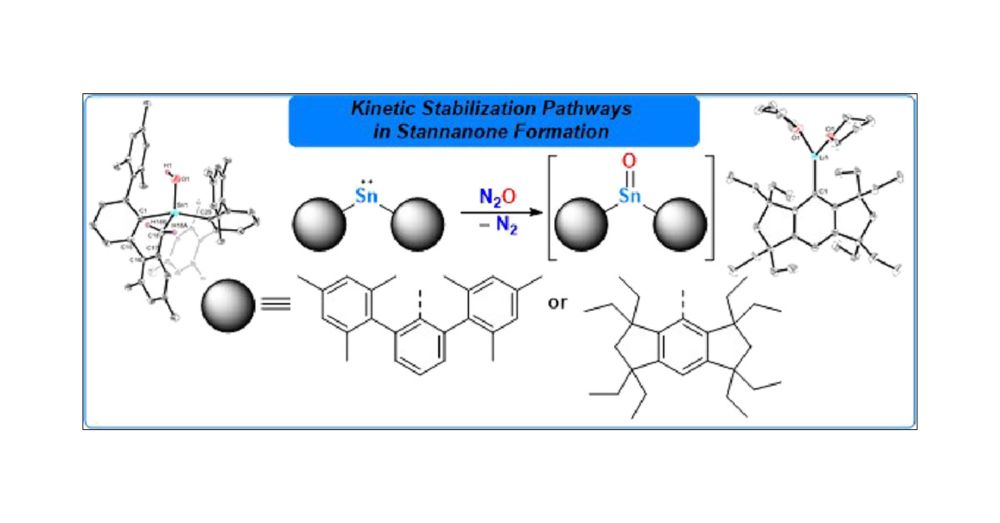
Kinetic Stabilization in Diaryl-Substituted Stannylenes: N2O Reactivity, Intramolecular C–H Activation, and Crystalline (Eind)Li(THF)2 as a Versatile Precursor in Tin Chemistry
The reactivity of the kinetically stabilized stannylene (MesTer)2Sn (1) (MesTer = –C6H3-2,6-(2,4,6-Me3-C6H2)2) toward N2O is revisited, yielding the terminal tin(IV) hydroxide 2 via formal intramolecular C(sp3)–H activation of a putative terminal stannanone intermediate. By switching to Eind ligation (Eind = 1,1,3,3,5,5,7,7-octaethyl-s-hydrindacen-4-yl) at the tin center, the synthesis and characterization of the crystalline lithium salt (Eind)Li(THF)2 (3) is reported, serving as a straightforward precursor for the clean generation of the corresponding stannylene (Eind)2Sn (4). Compound 4 can be further cleanly converted into the heteroleptic Eind/halide stannylene (Eind)SnCl (6). Both 4 and 6 serve as suitable precursors for the synthesis of the heteroleptic s-hydrindacene-/amido-substituted stannylene (Eind)Sn{N(SiMe3)2} (5).
pubs.acs.org
Reposted by Josh Abbenseth
Josh Abbenseth
@joshabbenseth.bsky.social
· Jun 26