Jaime Martín
@jaimechem.bsky.social
33 followers
36 following
3 posts
Research Chemist. Postdoc at the University of Zurich
Posts
Media
Videos
Starter Packs
Reposted by Jaime Martín
Reposted by Jaime Martín
Carole Duboc
@caroleduboc.bsky.social
· Mar 4
Interrogating the anti-Insertion of Alkynes into Gold(III)
Alkyne hydrofunctionalizations are a powerful strategy to efficiently build up structural complexity. The selectivity of these reactions is typically governed by the interaction between the alkyne and...
pubs.acs.org
Jaime Martín
@jaimechem.bsky.social
· Feb 28

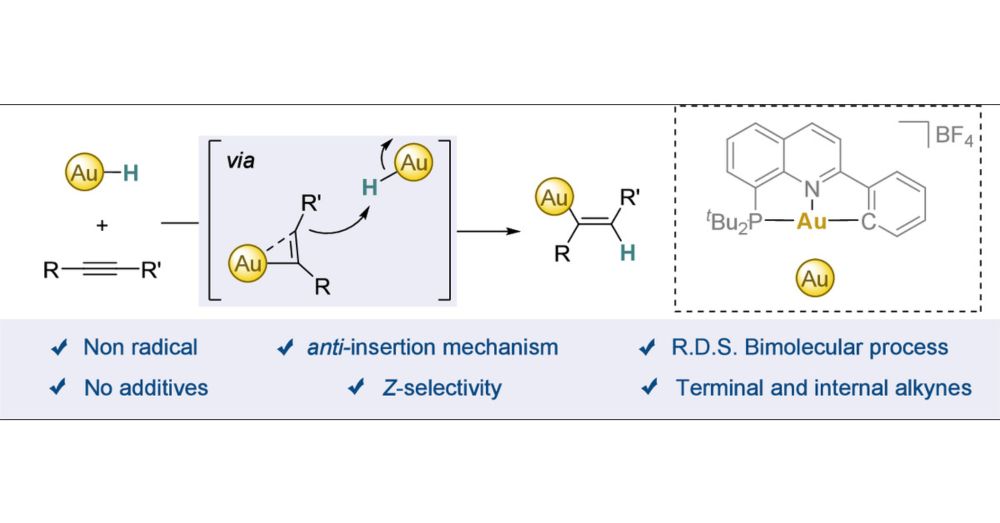
Interrogating the anti-Insertion of Alkynes into Gold(III)
Alkyne hydrofunctionalizations are a powerful strategy to efficiently build up structural complexity. The selectivity of these reactions is typically governed by the interaction between the alkyne and a metal-hydride, which commonly proceeds via a well-understood syn-insertion mechanism. In contrast, anti-insertions are far less common, with proposed mechanisms often extrapolated from literature precedents rather than grounded in direct experimental evidence. While gold complexes rank among the most efficient catalysts for such transformations, the mechanistic understanding of the key alkyne insertion step remains incomplete. In this study, we demonstrate that stable gold(III)-hydrides, featuring a (P∧N∧C) ligand, undergo selective insertion of alkynes to yield the corresponding anti-Markovnikov Z-vinyl complexes. A combination of control experiments, kinetic studies, and computational analyses reveals a nonradical, bimolecular insertion process, in which water plays a pivotal role by accelerating the reaction and potentially stabilizing a highly reactive, T-shaped gold(I) intermediate. Notably, this is the first demonstration of the insertion of both activated and unactivated terminal and internal alkynes into a gold(III)-hydride complex.
pubs.acs.org


