

If you’re interested in working out the mechanism and physiological impact of bacterial lipid transport processes then please apply!
Job advert is here: tinyurl.com/4swddfda
Get in touch by email ([email protected]) for informal enquiries
Please repost!
Thanks!
If you’re interested in working out the mechanism and physiological impact of bacterial lipid transport processes then please apply!
Job advert is here: tinyurl.com/4swddfda
Get in touch by email ([email protected]) for informal enquiries
Please repost!
Thanks!
Using #cryoEM we discovered unique features of the #Pseudomonas aeruginosa ATP synthase including an unexpected role for #zinc in the complex 🦠🔬
Using #cryoEM we discovered unique features of the #Pseudomonas aeruginosa ATP synthase including an unexpected role for #zinc in the complex 🦠🔬
🔗 www.cell.com/structure/fu...
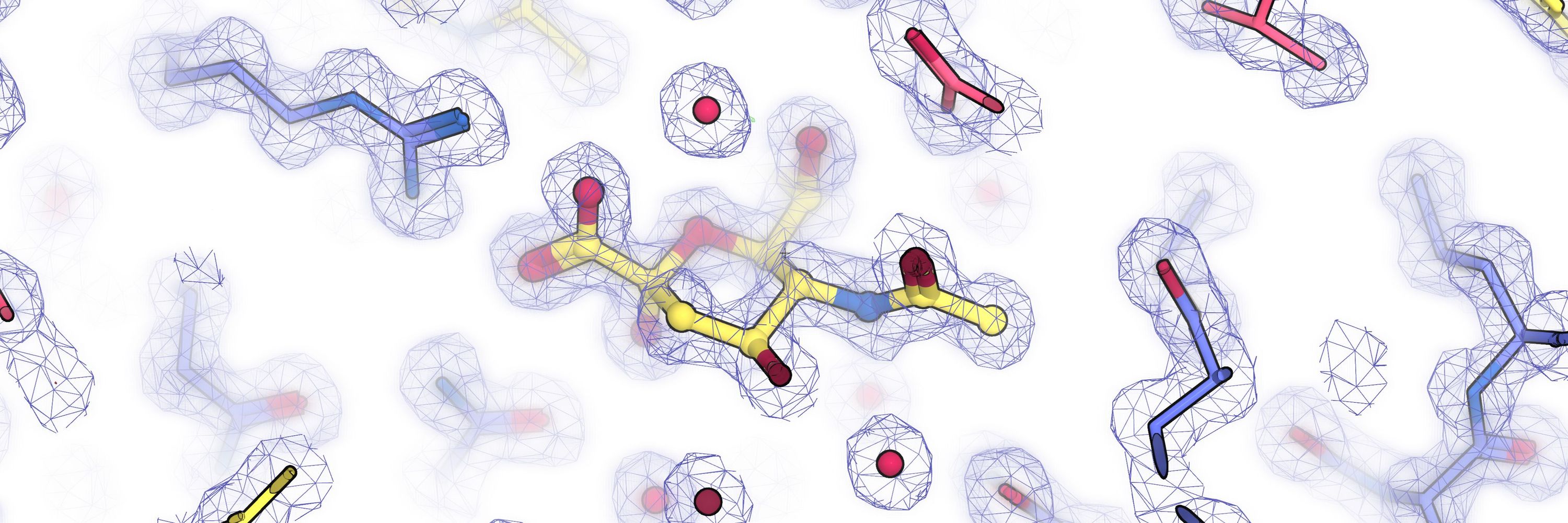
We explored how sulfate-reducing bacteria import isethionate, a sulfur-containing molecule found in the environment and produced by microbes in the human gut. We captured a structure of the IseQM TRAP transporter in a substrate-bound state.

🔗 www.cell.com/structure/fu...
We explored how sulfate-reducing bacteria import isethionate, a sulfur-containing molecule found in the environment and produced by microbes in the human gut. We captured a structure of the IseQM TRAP transporter in a substrate-bound state.
www.cell.com/structure/fu...

www.cell.com/structure/fu...

