Jesse Erasmus
@jesseerasmus.bsky.social
2.3K followers
1.1K following
160 posts
Director of Virology at HDT Bio. Developing RNA platform for emerging infectious diseases. 🇿🇦🇰🇷
Posts
Media
Videos
Starter Packs
Pinned
Jesse Erasmus
@jesseerasmus.bsky.social
· Nov 10
Jesse Erasmus
@jesseerasmus.bsky.social
· Dec 20

Replicating RNA vaccine confers durable immunity against Crimean Congo hemorrhagic fever virus challenge in mice - npj Vaccines
npj Vaccines - Replicating RNA vaccine confers durable immunity against Crimean Congo hemorrhagic fever virus challenge in mice
www.nature.com
Jesse Erasmus
@jesseerasmus.bsky.social
· Jun 19

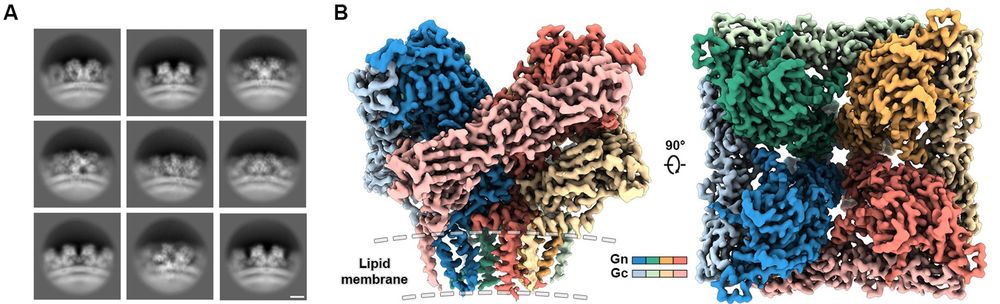
Antigen-dependent interplay of formulation, systemic innate responses, and antibody responses to multi-component replicon RNA vaccination
Lipid nanoparticles (LNPs) are a modular, non-viral nucleic acid delivery system.
Here, Eygeris and colleagues report that addition of saponin molecules, such as quillaic
acid, to LNP formulations can...
www.cell.com
Jesse Erasmus
@jesseerasmus.bsky.social
· Jun 19
Reposted by Jesse Erasmus
Jesse Erasmus
@jesseerasmus.bsky.social
· Jun 13
Reposted by Jesse Erasmus
Jesse Erasmus
@jesseerasmus.bsky.social
· May 21
Reposted by Jesse Erasmus