John Seed
@john-seed.bsky.social
79 followers
96 following
17 posts
Research Fellow in the Liddle group at the University of Manchester. Actinide metal-metal bonding, actinide-pnictogen chemistry, and actinide magnetism ☢️☢️🧲
Posts
Media
Videos
Starter Packs
Pinned
John Seed
@john-seed.bsky.social
· Apr 11
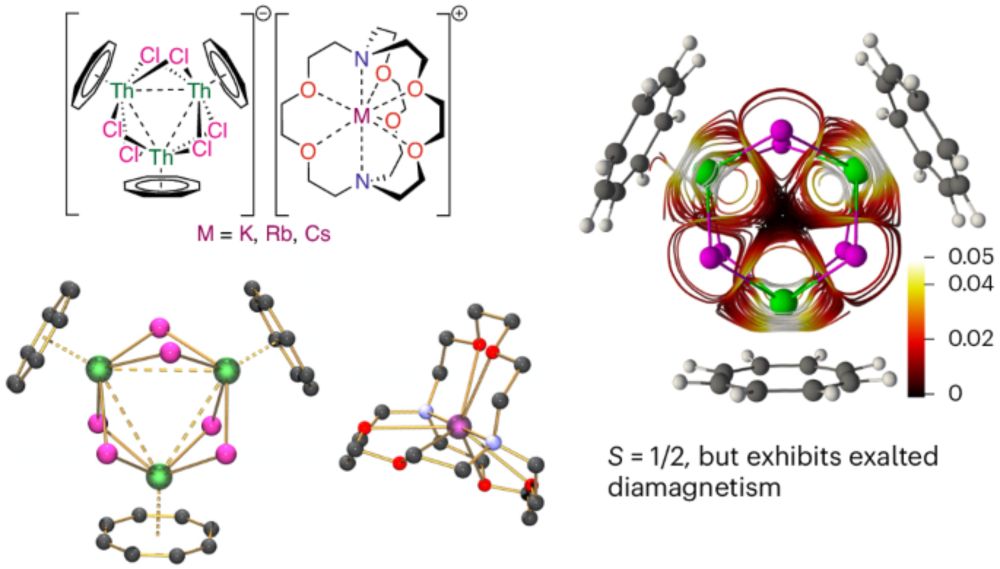
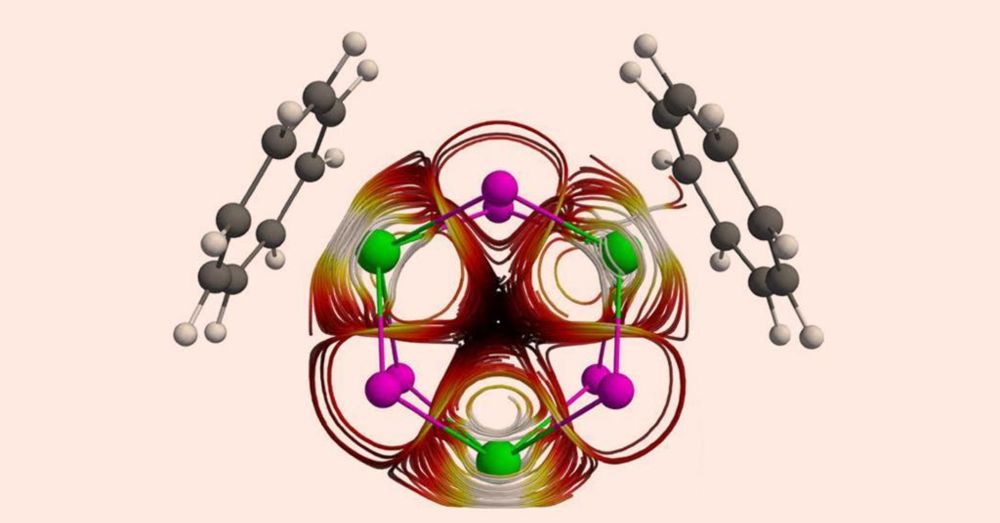
Valence-delocalized trithorium nanocluster superatoms with open-shell exalted diamagnetism - Nature Chemistry
Superatoms are metal clusters that collectively behave like an atom, but they usually require metal–metal bonding and thus they are based on main group or transition metals. Now it has been shown that...
www.nature.com
John Seed
@john-seed.bsky.social
· Aug 15
John Seed
@john-seed.bsky.social
· Aug 15

Uranium-stibinidiide, -stibinidene, and -stibido multiple bonds and uranium-nitride formation from multimetallic diuranium-distibene-mediated dinitrogen cleavage - Nature Communications
Although uranium-nitrogen multiple bonding is well developed, there are far fewer uranium-phosphorus and -arsenic multiple bonds, and none for antimony, even in spectroscopic scenarios. Here, the auth...
www.nature.com
John Seed
@john-seed.bsky.social
· Jul 17
John Seed
@john-seed.bsky.social
· Jul 17
John Seed
@john-seed.bsky.social
· Jul 10
Reposted by John Seed
David Mills
@millsgroupchem.bsky.social
· Jun 26

Magnetic hysteresis up to 73 K in a dysprosium cyclopentadienyl-amide single-molecule magnet
Single-molecule magnets (SMMs) based on dysprosocenium cations, [Dy(CpR)2]+ (CpR = substituted cyclopentadienyl), have set record effective energy barriers to magnetic reversal (Ueff) and temperatures...
chemrxiv.org
John Seed
@john-seed.bsky.social
· Jun 26
Reposted by John Seed
David Mills
@millsgroupchem.bsky.social
· Jun 25
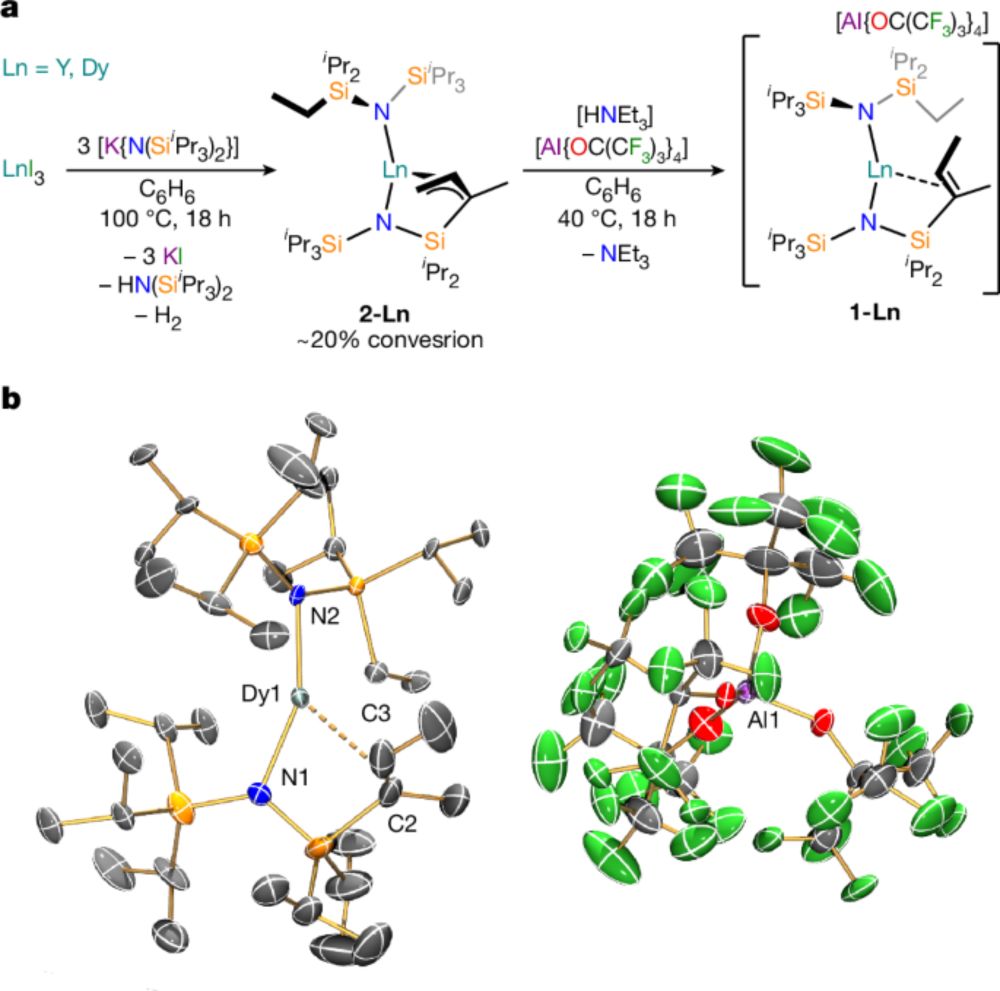
Soft magnetic hysteresis in a dysprosium amide–alkene complex up to 100 kelvin - Nature
A dysprosium amide–alkene complex shows soft magnetic hysteresis loops up to 100 kelvin, arising from the high charge density of the amide ligands and the structural role of the pendant alkene.
www.nature.com
John Seed
@john-seed.bsky.social
· May 30
John Seed
@john-seed.bsky.social
· Apr 17
John Seed
@john-seed.bsky.social
· Apr 17
John Seed
@john-seed.bsky.social
· Apr 17
John Seed
@john-seed.bsky.social
· Apr 11
John Seed
@john-seed.bsky.social
· Apr 11
John Seed
@john-seed.bsky.social
· Apr 11
John Seed
@john-seed.bsky.social
· Apr 11

Valence-delocalized trithorium nanocluster superatoms with open-shell exalted diamagnetism - Nature Chemistry
Superatoms are metal clusters that collectively behave like an atom, but they usually require metal–metal bonding and thus they are based on main group or transition metals. Now it has been shown that...
www.nature.com
John Seed
@john-seed.bsky.social
· Apr 9
John Seed
@john-seed.bsky.social
· Mar 1
Jake Yeston
@jakeyeston.bsky.social
· Feb 27

Berkelium–carbon bonding in a tetravalent berkelocene
Interest in actinide–carbon bonds has persisted since actinide organometallics were first investigated for applications in isotope separation during the Manhattan Project. Transplutonium organometalli...
www.science.org
Reposted by John Seed
Josef Boronski
@josefboronski.bsky.social
· Feb 17