Kay Severin
@kay-severin.bsky.social
1.2K followers
950 following
20 posts
Professor of chemistry at the EPFL, Switzerland
lcs.epfl.ch
Posts
Media
Videos
Starter Packs
Reposted by Kay Severin
Reposted by Kay Severin
Waser Group
@lcsolab.bsky.social
· Aug 30

Peptide/Protein Functionalization and Macrocyclization via Alkyne Umpolung with Hypervalent Iodine Reagents
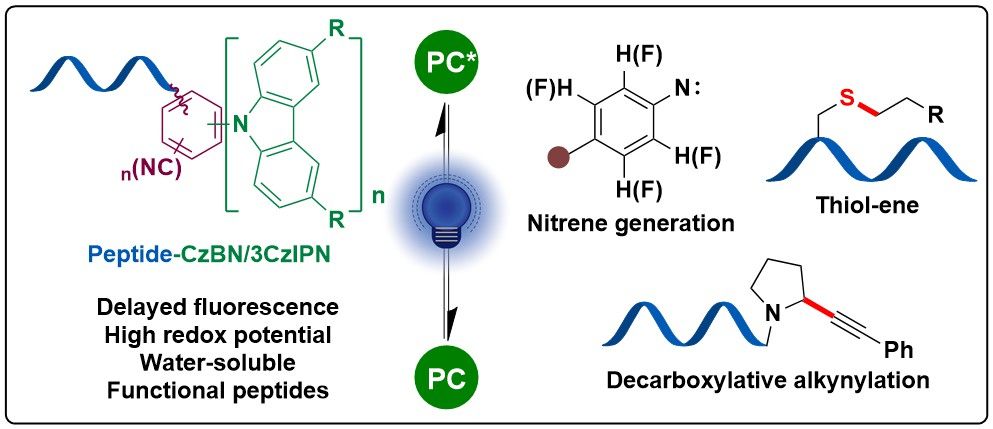
ConspectusAlkynes are one of the most fundamental functional groups in organic synthesis due to the versatile chemistry of the triple bond, their unique rigid structure, and their use in bioconjugation. The introduction of alkynes onto organic molecules traditionally relies on nucleophilic activation, often requiring strong bases or metal catalysts. These conditions, however, restrict applications involving biomolecules such as peptides and proteins due to functional group incompatibility. To address this limitation, our group developed an “umpolung” approach, utilizing hypervalent iodine compounds to create electrophilic alkyne transfer reagents such as benziodoxol(on)es (Bx(X)s) and benziodazolones (BZs). The high reactivity of EBx/X/Z reagents enables efficient alkyne transfer to various nucleophilic residues in peptides and proteins under different reaction conditions, providing a versatile tool for biomolecule modification.In this Account, we highlight the residue-selective alkynylation and alkenylation of peptides enabled by the development of novel EBx/X/Z reagents with a focus on progress since 2021. This includes the following: (1) Selective residue modification: We have made significant progress in the residue-selective alkynylation and alkenylation of peptides and proteins. Building on our initial work with Cys-selective alkynylation, we enhanced reactivity and solubility by introducing a sulfonate group on the benziodoxolone arene core, facilitating lipophilic alkynylation in an aqueous environment. Furthermore, we developed perfluoroaryl-modified BZ reagents to achieve sequential Cys-Cys cross-linking and used them for antibody cross-linking with superior reactivity compared to that of conventional methods. Additionally, we expanded the reactivity beyond Cys to achieve Tyr-selective conjugation. All of these achievements underscored the tunability of EBx/X/Z reagents through strategic substituent modification on the iodine core. (2) Peptide stapling and macrocyclization: We designed EBx(X) reagents featuring an additional reactive site on the alkyne moiety, enabling Cys-Cys and Cys-Lys stapling in peptides. This approach enhanced their α-helicity and potential as PPI inhibitors with improved binding affinity to the MDM2 protein. For sequences lacking Cys, we incorporated the whole EBx(X) core onto Lys residues via an activated ester on the alkyne, forming peptide-EBx(X) conjugates. These conjugates facilitated the formation of rigid, functional peptide macrocycles using C-terminal or Trp-selective alkynylation. The utility of these macrocyclizations was demonstrated by achieving improved binding affinity to the KEAP1 protein and by generating fluorescent cyclic peptides suitable for live-cell imaging without additional fluorophores. (3) Broadening applicability with EBx-containing amino acids: We prepared EBx amino acids compatible with both solid-phase peptide synthesis (SPPS) and solution-phase synthesis (SPS), allowing us to apply our cyclization strategies to construct a diverse library of cyclic peptides.
doi.org
Reposted by Kay Severin
Kay Severin
@kay-severin.bsky.social
· Aug 13
Kay Severin
@kay-severin.bsky.social
· Aug 13
Reposted by Kay Severin
Reposted by Kay Severin
Adrian Chaplin
@chaplinlab.bsky.social
· Jul 15
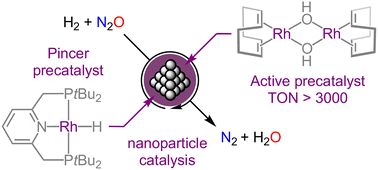
Rhodium-catalysed hydrogenation of nitrous oxide
We report on the discovery of “hidden” heterogeneous catalysis in the hydrogenation of nitrous oxide while assessing the catalytic activity of a rhodium(i) hydride complex supported by a nominally rob...
doi.org
Kay Severin
@kay-severin.bsky.social
· May 30
Kay Severin
@kay-severin.bsky.social
· May 30
Reposted by Kay Severin
Reposted by Kay Severin
Waser Group
@lcsolab.bsky.social
· May 25
Reposted by Kay Severin
Reposted by Kay Severin
Reposted by Kay Severin
Reposted by Kay Severin
Kay Severin
@kay-severin.bsky.social
· Mar 18