Laura Corrales
@lauracorrales.bsky.social
110 followers
250 following
7 posts
Molecular microbiologist
Ramón y Cajal Group Leader at unisevilla
https://www.ibvf.us-csic.es/
Posts
Media
Videos
Starter Packs
Laura Corrales
@lauracorrales.bsky.social
· Aug 27
Laura Corrales
@lauracorrales.bsky.social
· Aug 27
Reposted by Laura Corrales
Zedler Lab
@synbiojazz.bsky.social
· Mar 6
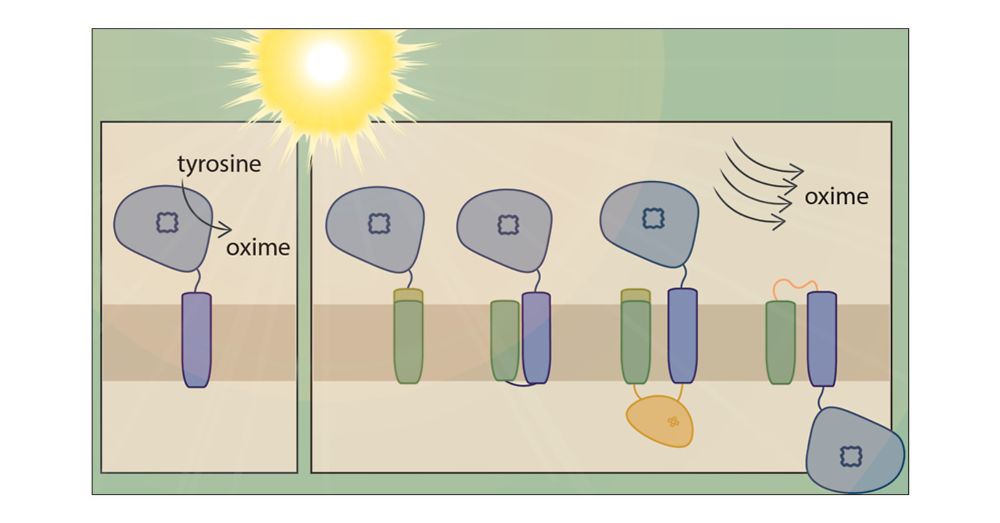
Thylakoid Targeting Improves Stability of a Cytochrome P450 in the Cyanobacterium Synechocystis sp. PCC 6803
Plants produce a large array of natural products of biotechnological interest. In many cases, these compounds are naturally produced at low titers and involve complex biosynthetic pathways, which often include cytochrome P450 enzymes. P450s are known to be difficult to express in traditional heterotrophic chassis. However, cyanobacteria have shown promise as a sustainable alternative for the heterologous expression of P450s and light-driven product biosynthesis. In this study, we explore strategies for improving plant P450 stability and membrane insertion in cyanobacteria. The widely used model cyanobacterium Synechocystis sp. PCC 6803 was chosen as the host, and the well-studied P450 CYP79A1 from the dhurrin pathway of Sorghum bicolor was chosen as the model enzyme. Combinations of the P450 fused with individual elements (e.g., signal peptide, transmembrane domain) or the full length cyanobacterial, thylakoid-localized, protein PetC1 were designed. All generated CYP79A1 variants led to oxime production. Our data show that strains producing CYP79A1 variants with elements of PetC1 improved thylakoid targeting. In addition, chlorophyll-normalized oxime levels increased, on average, up to 18 times compared to the unmodified CYP79A1. These findings offer promising strategies to improve heterologous P450 expression in cyanobacteria and can ultimately contribute to advancing light-driven biocatalysis in cyanobacterial chassis.
pubs.acs.org
Reposted by Laura Corrales






