Lisa Gourdon-Grünewaldt
@lggchem.eu
1.5K followers
1K following
6 posts
Postdoc DNA mimics 🧫🧬 @akhuc.bsky.social at LMU | PhD photodynamic therapy 🧪💡 @gassergroup.bsky.social & @cariouk.bsky.social at PSL | Alumna Chimie ENS Ulm | 🌈🚲🛠️
Member of Young French ChemBio fychembio.fr
lggchem.eu #ChemBio
ORCID: 0000-0003-0932-7838
Posts
Media
Videos
Starter Packs
Reposted by Lisa Gourdon-Grünewaldt
Reposted by Lisa Gourdon-Grünewaldt
Reposted by Lisa Gourdon-Grünewaldt
Lisa Gourdon-Grünewaldt
@lggchem.eu
· Jun 10
Reposted by Lisa Gourdon-Grünewaldt
Ivan Huc Group
@akhuc.bsky.social
· Apr 15

Development of Aromatic Foldamer Building Blocks Bearing Multiple Biogenic Side Chains
Aromatic oligoamides, with their intrinsic rigidity and well-defined conformations, are recognized for their potential in medical applications. Similar structures are present in several naturally occurring antibiotics and have been explored for their ability to bind to various proteins and B-DNA (canonical right-handed DNA helix). This study introduces a synthetic approach to produce quinoline amino acid monomers bearing diversified side chain combinations in positions 4, 5, and 6 of the quinoline ring, designed to enhance the side chain density on helical foldamers. By increasing the number of side chains on each monomer, we aim to mimic the dense side chain presentation of α-peptides, thus improving the potential for protein surface recognition. This synthetic strategy involves efficient functionalization through cross-coupling reactions, enabling the installation of diverse side chains at strategic positions on the quinoline ring. The process has been optimized for automated solid-phase synthesis, successfully producing a 20-unit oligoamide with good purity. This foldamer, featuring multiple cationic, anionic, polar, and hydrophobic side chains, demonstrates the potential for molecular recognition in drug discovery and therapeutic applications. The methodology described here represents a significant advancement in the construction of aromatic oligoamide foldamers, providing a robust platform for further exploration of biological systems.
pubs.acs.org
Reposted by Lisa Gourdon-Grünewaldt
Lisa Gourdon-Grünewaldt
@lggchem.eu
· Feb 27
Ivan Huc Group
@akhuc.bsky.social
· Feb 27
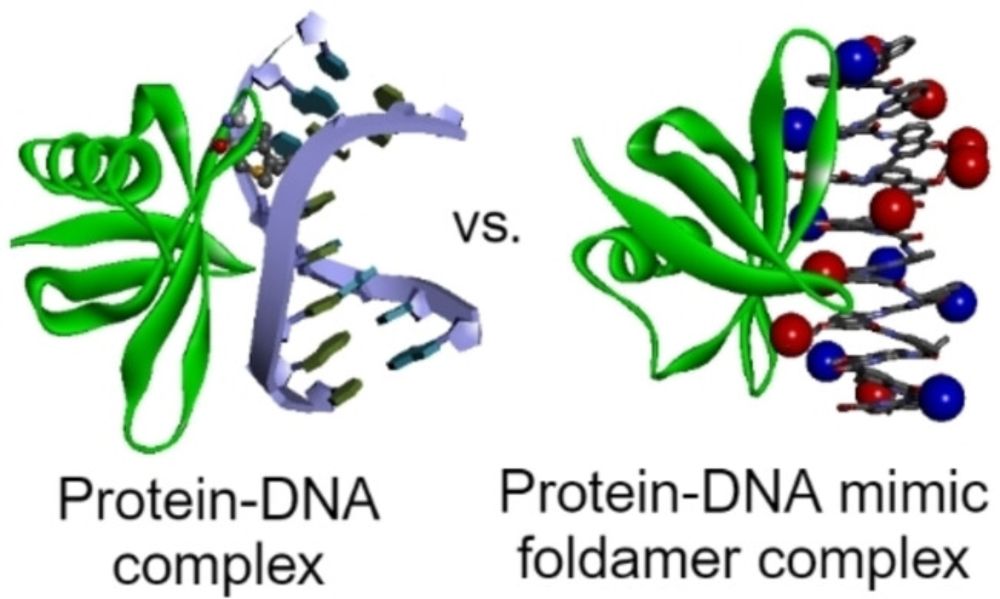
DNA Mimic Foldamer Recognition of a Chromosomal Protein
Biomolecular mimics are intended to outperform their natural counterparts. However, B-DNA surface mimicry to target DNA-binding proteins has long remained underdeveloped. We have introduced helical a...
onlinelibrary.wiley.com
Reposted by Lisa Gourdon-Grünewaldt
Ivan Huc Group
@akhuc.bsky.social
· Dec 27

Interrogating the potential of helical aromatic foldamers for protein recognition
A biotinylated helical aromatic oligoamide foldamer equivalent in size to a 24mer peptide was designed without any prejudice other than to display various polar and hydrophobic side chains at its surf...
pubs.rsc.org
Reposted by Lisa Gourdon-Grünewaldt
Reposted by Lisa Gourdon-Grünewaldt
Reposted by Lisa Gourdon-Grünewaldt
Reposted by Lisa Gourdon-Grünewaldt