Melé Lab
@melelab.bsky.social
150 followers
250 following
24 posts
We are the Melé Lab, the Transcriptomics and Functional Genomics Lab (TFGL) at the Barcelona Supercomputing Center (BSC).
Account by lab members
Posts
Media
Videos
Starter Packs
Melé Lab
@melelab.bsky.social
· May 23
Reposted by Melé Lab
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20

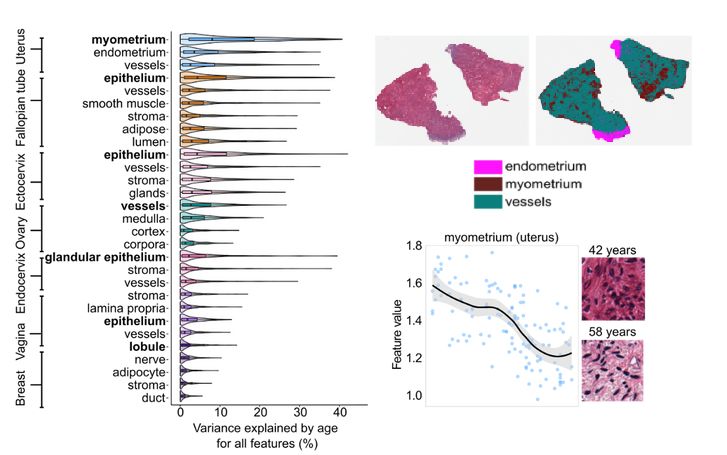
Long-read transcriptomics of a diverse human cohort reveals widespread ancestry bias in gene annotation
Accurate gene annotations are fundamental for interpreting genetic variation, cellular function, and disease mechanisms. However, current human gene annotations are largely derived from transcriptomic...
www.biorxiv.org
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20
Melé Lab
@melelab.bsky.social
· Mar 20

Long-read transcriptomics of a diverse human cohort reveals widespread ancestry bias in gene annotation
Accurate gene annotations are fundamental for interpreting genetic variation, cellular function, and disease mechanisms. However, current human gene annotations are largely derived from transcriptomic...
www.biorxiv.org