Philip
@philiplampkin.bsky.social
61 followers
130 following
9 posts
Graduate student in the Gellman Lab (UW–Madison). Incoming postdoc in the Sigman Group (Utah). Physical Organic Chemist.
Posts
Media
Videos
Starter Packs
Pinned
Philip
@philiplampkin.bsky.social
· Sep 5

Bifunctional Catalysis of Aldol Reactions by Foldamer Dihydrazides: Assessment of Conformational Preorganization
We previously reported that molecules containing two cyclic hydrazide units connected by a polymethylene linker could catalyze aldol condensations via a bifunctional mechanism. One hydrazide apparentl...
doi.org
Philip
@philiplampkin.bsky.social
· Sep 5

Bifunctional Catalysis of Aldol Reactions by Foldamer Dihydrazides: Assessment of Conformational Preorganization
We previously reported that molecules containing two cyclic hydrazide units connected by a polymethylene linker could catalyze aldol condensations via a bifunctional mechanism. One hydrazide apparentl...
doi.org
Reposted by Philip
Reposted by Philip
Jeff Martell
@jeffmartell.bsky.social
· Aug 14
DNA-Scaffolded Ultrahigh-Throughput Reaction Screening
Discovering and optimizing reactions is central to synthetic chemistry. However, chemical reactions are traditionally screened using relatively low-throughput methods, prohibiting exploration of diver...
chemrxiv.org
Reposted by Philip
Philip
@philiplampkin.bsky.social
· Jul 8
Philip
@philiplampkin.bsky.social
· Jul 8

Versatile Open-Source Photoreactor Architecture for Photocatalysis Across the Visible Spectrum
Adoption of commercial photoreactors as standards for photocatalysis research could be limited by high cost. We report the development of the Wisconsin Photoreactor Platform (WPP), an open-source phot...
doi.org
Philip
@philiplampkin.bsky.social
· Jun 2
Reposted by Philip
Brenna Bierman
@brennabierman.bsky.social
· Mar 13
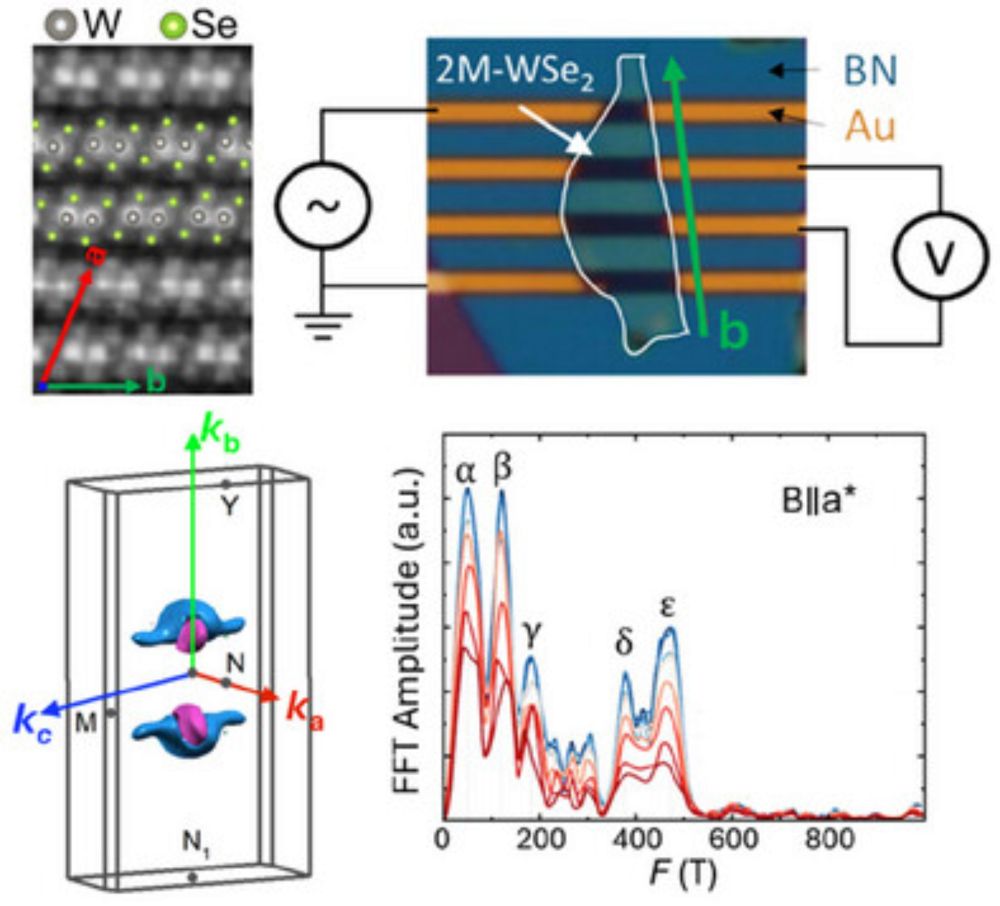
Evidence for Topological States and a Lifshitz Transition in Metastable 2M‐WSe2
This study demonstrates the Fermi surface characteristics and transport properties of 2M-WSe2 bulk single crystals. The measurements and first-principles calculations reveal multiple Fermi pockets wi...
doi.org
Reposted by Philip
Weix Group
@weixgroup.bsky.social
· Mar 7
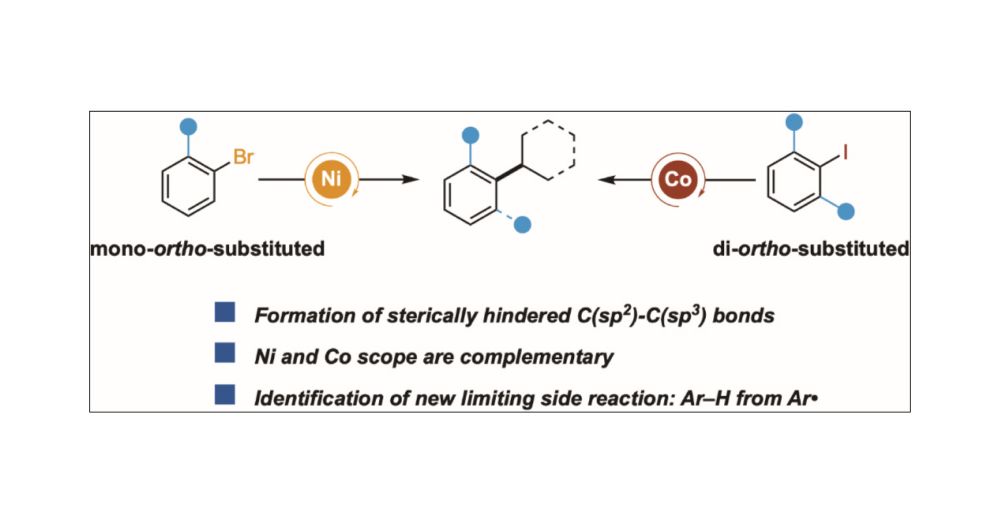
Cross-Electrophile Coupling to Form Sterically Hindered C(sp2)–C(sp3) Bonds: Ni and Co Afford Complementary Reactivity
The formation of sterically hindered C(sp2)–C(sp3) bonds could be a useful synthetic tool but has been understudied in cross-electrophile coupling. Here, we report two methods that couple secondary alkyl bromides with aryl halides that contain sterically hindered C–X bonds: 1) ortho-substituted aryl bromides with nickel catalysts and 2) di-ortho-substituted aryl iodides with cobalt catalysts. Stoichiometric experiments and deuterium labeling studies show that 1) [Co] is better than [Ni] for oxidative addition of di-ortho-substituted Ar–I and 2) [Co] is better than [Ni] for radical capture/reductive elimination steps with di-ortho-substituted arenes. For both metals, Ar–H side products observed in reactions with low-yielding di-ortho-substituted aryl iodides appear to arise from Ar• formation and hydrogen-atom transfer from the solvent. While the origins of the differences in scope are not yet understood, these studies demonstrate a previously unknown complementarity between nickel and cobalt in cross-electrophile coupling.
doi.org
Reposted by Philip
Martina Delbianco
@delbiancom.bsky.social
· Feb 26

A glycan foldamer that uses carbohydrate–aromatic interactions to perform catalysis - Nature Chemistry
In nature, catalysis is generally performed by proteins and ribozymes. Now it has been shown that glycans can be designed to perform catalysis. Exploiting carbohydrate–aromatic interactions, a glycan ...
www.nature.com
Reposted by Philip
Philip
@philiplampkin.bsky.social
· Jan 15
Philip
@philiplampkin.bsky.social
· Jan 15
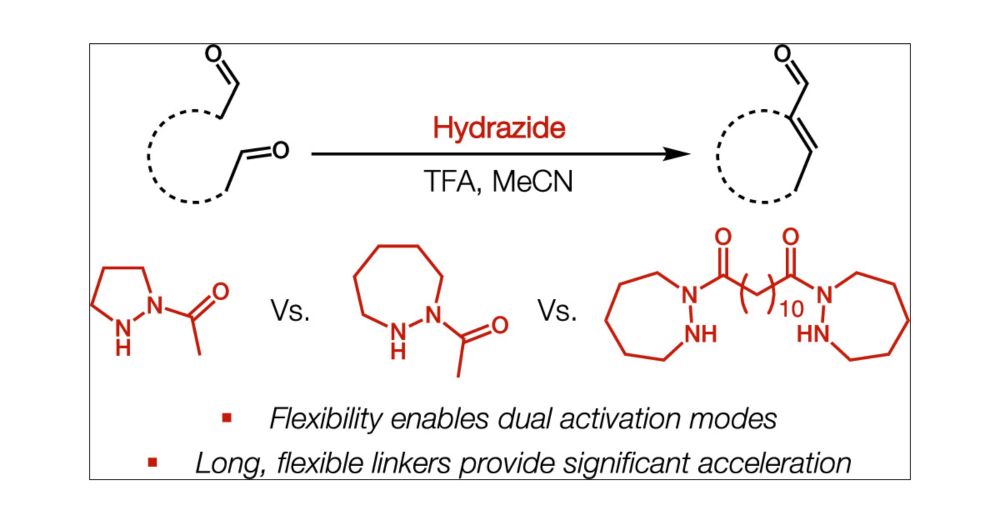
Dual Activation Modes Enable Bifunctional Catalysis of Aldol Reactions by Flexible Dihydrazides
Hydrazides are known to catalyze reactions of α,β-unsaturated aldehydes via transient iminium formation. The iminium intermediate displays enhanced electrophilicity, which facilitates conjugate additi...
doi.org
Reposted by Philip
Zach Wickens
@zachwickens.bsky.social
· Dec 12

Alkene Carboxy-Alkylation via CO2•–
Herein, we introduce a new platform for alkene carboxy-alkylation. This reaction is designed around CO2•– addition to alkenes followed by radical polar crossover, which enables alkylation through carbanion attack on carbonyl electrophiles. We discovered that CO2•– adds to alkenes faster than it reduces carbonyl electrophiles and that this reactivity can be exploited by accessing CO2•– via hydrogen atom transfer from formate. This photocatalytic system transforms vinylarenes and carbonyl compounds into a diverse array of substituted γ-lactone products. Furthermore, indoles can be engaged through dearomative carboxy-alkylation, delivering medicinally relevant C(sp3)-rich heterocyclic scaffolds. Mechanistic studies reveal that the active photocatalyst is generated in situ through a photochemically induced reaction between the precatalyst and DMSO. Overall, we have developed a three-component alkene carboxy-alkylation reaction enabled by the use of formate as the CO2•– precursor.
pubs.acs.org
Reposted by Philip
Eszter Boros
@eboros.bsky.social
· Nov 21







