


chemsky 🧪
www.science.org/doi/10.1126/...

chemsky 🧪
www.science.org/doi/10.1126/...

chemsky 🧪
www.science.org/doi/10.1126/...
100 ns lifetime, 3 eV energy storage, and 37% quantum yield
A step toward multi-electron photochemistry
Mathis Brändlin and @bjoernpfund.bsky.social in @natchem.nature.com
www.nature.com/articles/s41...

100 ns lifetime, 3 eV energy storage, and 37% quantum yield
A step toward multi-electron photochemistry
Mathis Brändlin and @bjoernpfund.bsky.social in @natchem.nature.com
www.nature.com/articles/s41...
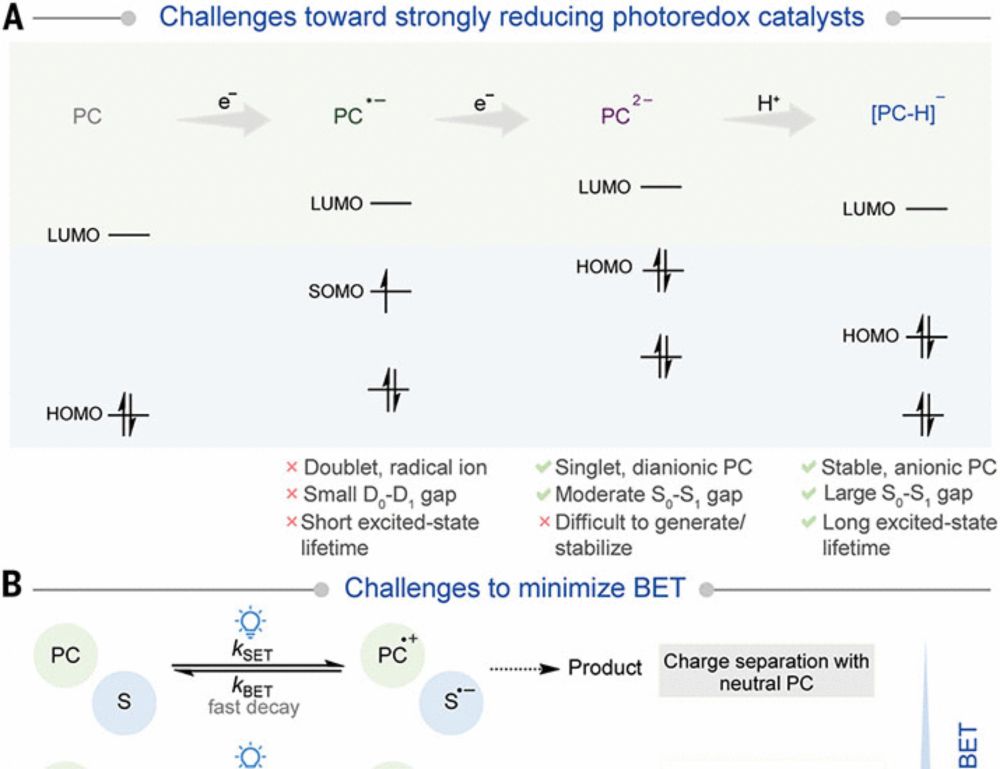







doi.org/10.1002/anie...
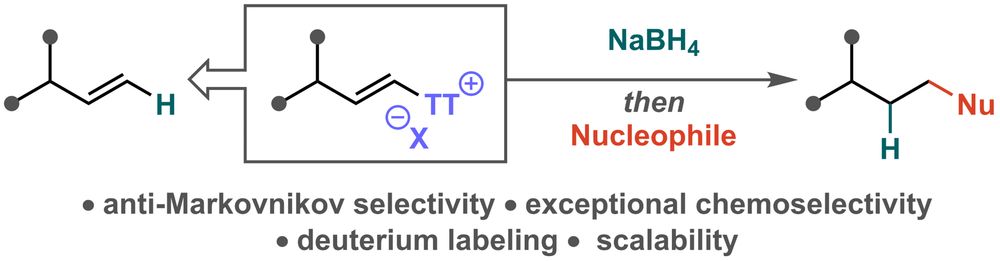
doi.org/10.1002/anie...
What follows will include some generalizations based on population averages of what I have experienced over the course of my career. There are, of course, exceptions in every group who are substantially more to one extreme or the other.

What follows will include some generalizations based on population averages of what I have experienced over the course of my career. There are, of course, exceptions in every group who are substantially more to one extreme or the other.
If you can't make it to your state capital JUST WALK OUT
standupforscience2025.org
standupforscience2025.org/organize-a-s...

If you can't make it to your state capital JUST WALK OUT
standupforscience2025.org
standupforscience2025.org/organize-a-s...
1616 – Galileo Galilei is formally banned by the Roman Catholic Church from teaching or defending the view that the earth orbits the sun.

1616 – Galileo Galilei is formally banned by the Roman Catholic Church from teaching or defending the view that the earth orbits the sun.
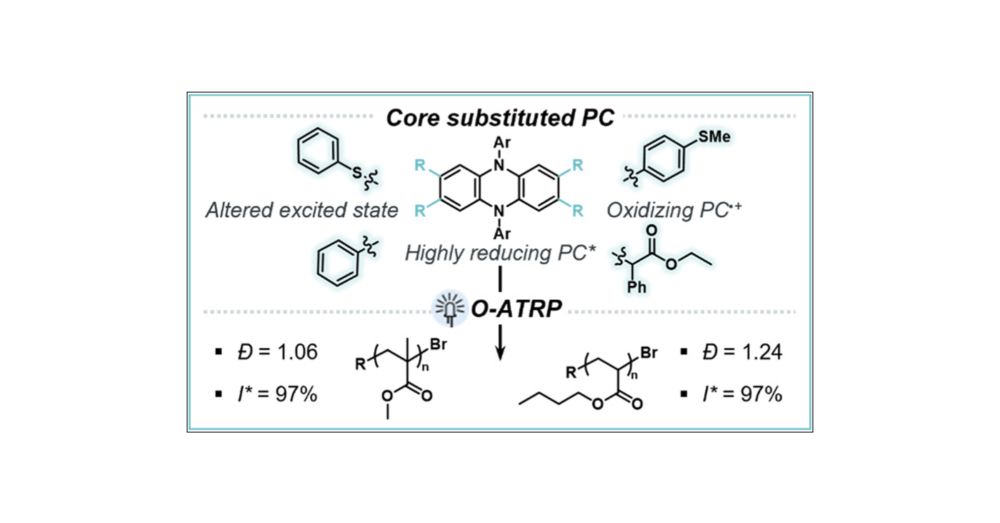
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/...
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/...

pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...



