Megan R. Hill
@drmrhill.bsky.social
Assistant Professor at Colorado State University. Enjoys polymer chemistry, tannic drinks, animals doing things, and learning how to mom.
Very excited to share our recent article in JACS where we showed that we could capture mechanoradicals formed during polymer degradation and use them to grow polymers back to high MWs or prime them for depolymerization! pubs.acs.org/doi/10.1021/...

Effective Recycling Pathways of Commodity Polymers Enabled by Mechanoradical Capture
Plastics pervade every aspect of modern life, yet effective mechanical recycling remains a major challenge. This is, in part, because of the mechanical forces that are involved in reprocessing, which break polymer chains and generate mechanoradicals, leading to a reduction in molecular weight and diminished material properties. This work introduces a robust strategy to capture and redirect these reactive intermediates, enabling value-preserving recycling pathways for widely used polymers polystyrene (PS) and poly(methyl methacrylate) (PMMA). By employing ball milling to induce chain scission, we demonstrate that mechanoradicals can be trapped by bis(butyl trithiocarbonate), yielding polymers with trithiocarbonate (TTC) end groups. Polymers degraded via ball milling showed significant reduction in molecular weight, ≈90% lower than the pristine polymers. These low molecular weight, TTC-functionalized polymers then served as macroinitiators for light-mediated controlled polymerization or, in the case of PMMA, as mediators for depolymerization under mild conditions. Chain extension of the degraded materials led to restored or increased molecular weight compared to the pristine polymers. Shear oscillatory rheology experiments revealed a recovery of entangled polymer properties, as evidenced by the reappearance of the rubbery plateau. We further showed that this “capture-and-repair” strategy is compatible with multiple cycles of degradation and chain extension, achieving repeated molecular weight recovery over three cycles. Additionally, we found that ball milling alone lowers the thermal depolymerization temperature of PMMA, enabling up to ≈44% depolymerization at 220 °C. Together, these findings highlight mechanoradical capture as a promising strategy to both enhance circularity and improve overall performance of mechanically recycled plastics.
pubs.acs.org
November 10, 2025 at 8:00 PM
Very excited to share our recent article in JACS where we showed that we could capture mechanoradicals formed during polymer degradation and use them to grow polymers back to high MWs or prime them for depolymerization! pubs.acs.org/doi/10.1021/...
Reposted by Megan R. Hill
Be my colleague! Cal Poly San Luis Obispo is hiring two tenure-track positions for Fall 2026, chemical education and coatings technology.
Chen Ed: jobs.calpoly.edu/en-us/job/55...
Coatings: jobs.calpoly.edu/en-us/job/55...
Chen Ed: jobs.calpoly.edu/en-us/job/55...
Coatings: jobs.calpoly.edu/en-us/job/55...
Cal Poly - Details - Tenure Track Position - Chemistry
jobs.calpoly.edu
September 17, 2025 at 6:05 PM
Be my colleague! Cal Poly San Luis Obispo is hiring two tenure-track positions for Fall 2026, chemical education and coatings technology.
Chen Ed: jobs.calpoly.edu/en-us/job/55...
Coatings: jobs.calpoly.edu/en-us/job/55...
Chen Ed: jobs.calpoly.edu/en-us/job/55...
Coatings: jobs.calpoly.edu/en-us/job/55...
Reposted by Megan R. Hill
Congrats to Amreen and Arindam and the whole team on getting their work out in Science today!! www.science.org/doi/10.1126/...
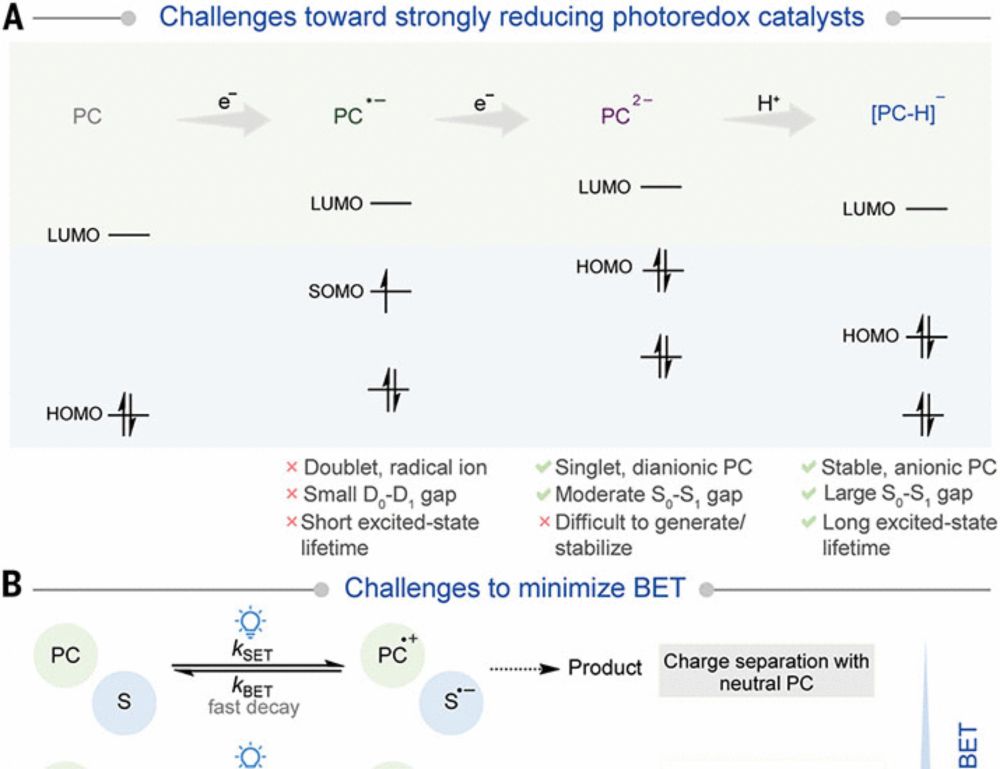
Efficient super-reducing organic photoredox catalysis with proton-coupled electron transfer mitigated back electron transfer
Photoredox catalysis driven by visible light has improved chemical synthesis by enabling milder reaction conditions and unlocking distinct reaction mechanisms. Despite the transformative impact, visib...
www.science.org
June 19, 2025 at 9:39 PM
Congrats to Amreen and Arindam and the whole team on getting their work out in Science today!! www.science.org/doi/10.1126/...
Reposted by Megan R. Hill
Congrats to former SuPRCat-er and recent grad Katrina on her publication in ACS Catalysis! pubs.acs.org/doi/10.1021/...
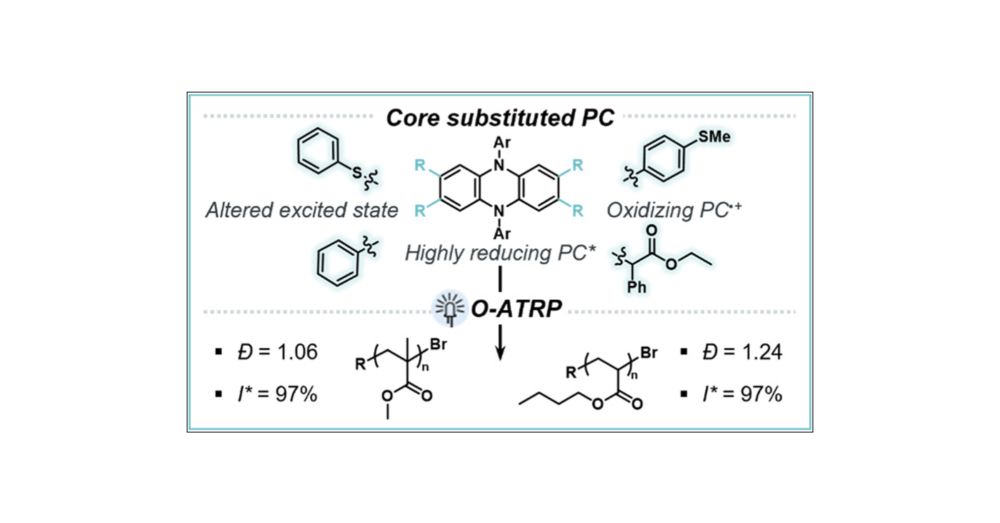
Influence of Dihydrophenazine Photoredox Catalyst Excited State Character and Reduction Potentials on Control in Organocatalyzed Atom Transfer Radical Polymerization
The development of N,N-diaryl dihydrophenazine organic photoredox catalysts (PCs) has enabled numerous examples of organocatalyzed atom transfer radical polymerization (O-ATRP) of methyl methacrylate (MMA) monomer to polymers with low dispersity (Đ < 1.30) and near-unity initiator efficiency (I* ∼ 100%), as well as small molecule synthesis. In this work, we investigate the influence of core substitution (CS) by alkyl, aryl, and heteroatom groups on singlet excited state reduction potential (ES1°*). We observe that a highly reducing ES1°* is in part a result of a locally excited (LE)-dominated hybridized local and charge transfer (HLCT) excited state in CS PCs, which is influenced by the identity of the core substituent. Additionally, the PCs that possess a LE-dominated HLCT character maintain a relatively oxidizing PC radical cation oxidation potential (E1/2) for deactivation in O-ATRP compared to fully LE PCs reported in prior work. For example, a thiophenol core substituted (heteroatom CS, HetCS) PC shows the most negative ES1°* (−2.07 V vs SCE), more LE character (Stokes shift = 124 nm), and has an oxidizing PC radical cation (E1/2 = 0.30 V vs SCE). The CS PCs with improved properties, including more negative ES1°*, perform best in O-ATRP of MMA with the HetCS PC showing the best control in both DMAc (Đ = 1.08, I* = 89%) and EtOAc (Đ = 1.06, I* = 97%). Additionally, the HetCS PC was found to mediate the controlled polymerization of n-butyl acrylate (n-BA) (Đ = 1.24, I* = 97%), which has remained challenging in O-ATRP without supplemental deactivation strategies. An aryl CS PC was found to have moderate control as low as 1 ppm PC, indicating facilitation of low PC loadings (Đ = 1.33, I* = 69%). The relationship between excited state character, ES1°*, and polymerization control observed in this work provides a foundation for increasing the utility of phenazine PCs across photoredox catalysis.
pubs.acs.org
March 11, 2025 at 9:59 PM
Congrats to former SuPRCat-er and recent grad Katrina on her publication in ACS Catalysis! pubs.acs.org/doi/10.1021/...
Reposted by Megan R. Hill
It may not be cool or hip in some circles, but we legitimately do need a massive amount of people to run for office.
Flood the school boards, councils, county seats, state legislatures, Congress, etc.
Big changes at the top happen when the ground shifts at the bottom. And that can start now.
Flood the school boards, councils, county seats, state legislatures, Congress, etc.
Big changes at the top happen when the ground shifts at the bottom. And that can start now.
February 22, 2025 at 8:07 PM
It may not be cool or hip in some circles, but we legitimately do need a massive amount of people to run for office.
Flood the school boards, councils, county seats, state legislatures, Congress, etc.
Big changes at the top happen when the ground shifts at the bottom. And that can start now.
Flood the school boards, councils, county seats, state legislatures, Congress, etc.
Big changes at the top happen when the ground shifts at the bottom. And that can start now.
Reposted by Megan R. Hill
On really sad lab days in grad school, I'd tell my labmates they were going to make a mistake, make a serendipitous discovery, and get a Science paper out of it.
For one chemist, that happened! An accidental discovery of a new way to recycle Plexiglas! 😍
via @bribarbu.bsky.social:
🧪⚗️ #chemsky
For one chemist, that happened! An accidental discovery of a new way to recycle Plexiglas! 😍
via @bribarbu.bsky.social:
🧪⚗️ #chemsky

Breaking down Plexiglas with light
Chlorine radicals unmake commercial polymethacrylates under mild conditions
cen.acs.org
February 20, 2025 at 11:52 PM
On really sad lab days in grad school, I'd tell my labmates they were going to make a mistake, make a serendipitous discovery, and get a Science paper out of it.
For one chemist, that happened! An accidental discovery of a new way to recycle Plexiglas! 😍
via @bribarbu.bsky.social:
🧪⚗️ #chemsky
For one chemist, that happened! An accidental discovery of a new way to recycle Plexiglas! 😍
via @bribarbu.bsky.social:
🧪⚗️ #chemsky
Reposted by Megan R. Hill
To do this & do it without any apparent input from longtime members (& to serve up an obnoxiously cutesy 404 page on top of it all) is very bad
The @acs.org has deleted its website on diversity, equity, inclusion and respect.
www.acs.org/about/divers...
www.acs.org/about/divers...
www.acs.org
February 10, 2025 at 12:26 PM
To do this & do it without any apparent input from longtime members (& to serve up an obnoxiously cutesy 404 page on top of it all) is very bad
I've been wondering when this would happen!
Yep! Sorry, I usually don't bother with the link bc of the paywall, but I should probably just drop it in the replies anyway

E&E News: Novel lawsuit targets chemical giants over recycling claims
A Kansas county is aiming to hold industry groups liable for plastic pollution stemming from misleading recyclability advertisements.
subscriber.politicopro.com
December 3, 2024 at 5:20 PM
I've been wondering when this would happen!
Reposted by Megan R. Hill
Every academic right now

November 29, 2024 at 9:58 PM
Every academic right now
Such cool work!! Did the Stache lab just solve the plastic crisis?? 😅 can’t wait to see what’s next!
Wouldn’t it be great if we could recycle plastic waste using sunlight? Now we can! Check out our latest work on recycling post-consumer polystyrene plastic! pubs.acs.org/doi/epdf/10....
#chemsky #bluesci #Sustainability #plastic #pollution @acspublications.bsky.social @stache-lab.bsky.social
#chemsky #bluesci #Sustainability #plastic #pollution @acspublications.bsky.social @stache-lab.bsky.social
Recycling of Post-Consumer Waste Polystyrene Using Commercial Plastic Additives
You have to enable JavaScript in your browser's settings in order to use the eReader.
pubs.acs.org
November 26, 2024 at 2:30 AM
Such cool work!! Did the Stache lab just solve the plastic crisis?? 😅 can’t wait to see what’s next!
Tons of great insight about microplastics in this edition of @cenmag.bsky.social but this one I found particularly interesting.
My latest feature for @cenmag.bsky.social examines biodegradable and compostable polymers as a solution to microplastic pollution.
Spoiler: While there are plenty of ways to use these materials smartly, microplastics are mostly a problem that new plastic can't solve!
cen.acs.org/materials/po...
Spoiler: While there are plenty of ways to use these materials smartly, microplastics are mostly a problem that new plastic can't solve!
cen.acs.org/materials/po...

Yes, biodegradable microplastics are a real thing
How long they last depends on where they end up
cen.acs.org
November 25, 2024 at 9:50 PM
Tons of great insight about microplastics in this edition of @cenmag.bsky.social but this one I found particularly interesting.
Reposted by Megan R. Hill
Cool work by Cassandra Callmann & team in ACS Cent Sci reporting a sugar-like polymer that removes heavy metals from water by forming a recyclable precipitate. As proof-of-concept, the polymer removed Cd & Pb from river water spiked with these persistent contaminants pubs.acs.org/doi/10.1021/...
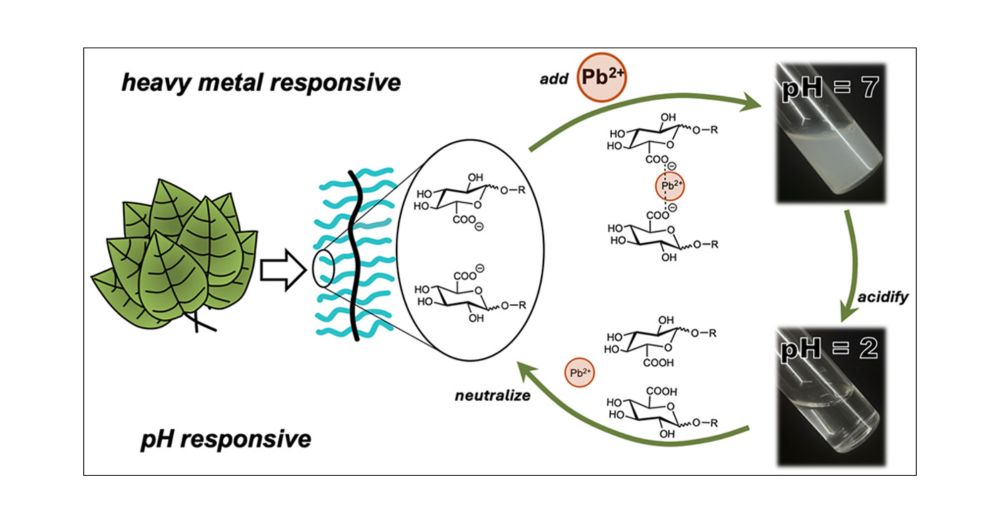
Bioinspired, Carbohydrate-Containing Polymers Efficiently and Reversibly Sequester Heavy Metals
Water scarcity and heavy metal pollution are significant challenges in today’s industrialized world. Conventional heavy metal remediation methods are often inefficient and energy-intensive, and produc...
pubs.acs.org
November 22, 2024 at 9:11 PM
Cool work by Cassandra Callmann & team in ACS Cent Sci reporting a sugar-like polymer that removes heavy metals from water by forming a recyclable precipitate. As proof-of-concept, the polymer removed Cd & Pb from river water spiked with these persistent contaminants pubs.acs.org/doi/10.1021/...
Reposted by Megan R. Hill
There are two really cool #PFAS / #foreverchemicals degradation papers out in Nature today
In one, the authors show that powdered Teflon can be transformed into charcoal+fluoride with light+a catalyst+reducing reagent & some very important help from mainly KOH and Cs formate
This is bonkers cool:
In one, the authors show that powdered Teflon can be transformed into charcoal+fluoride with light+a catalyst+reducing reagent & some very important help from mainly KOH and Cs formate
This is bonkers cool:
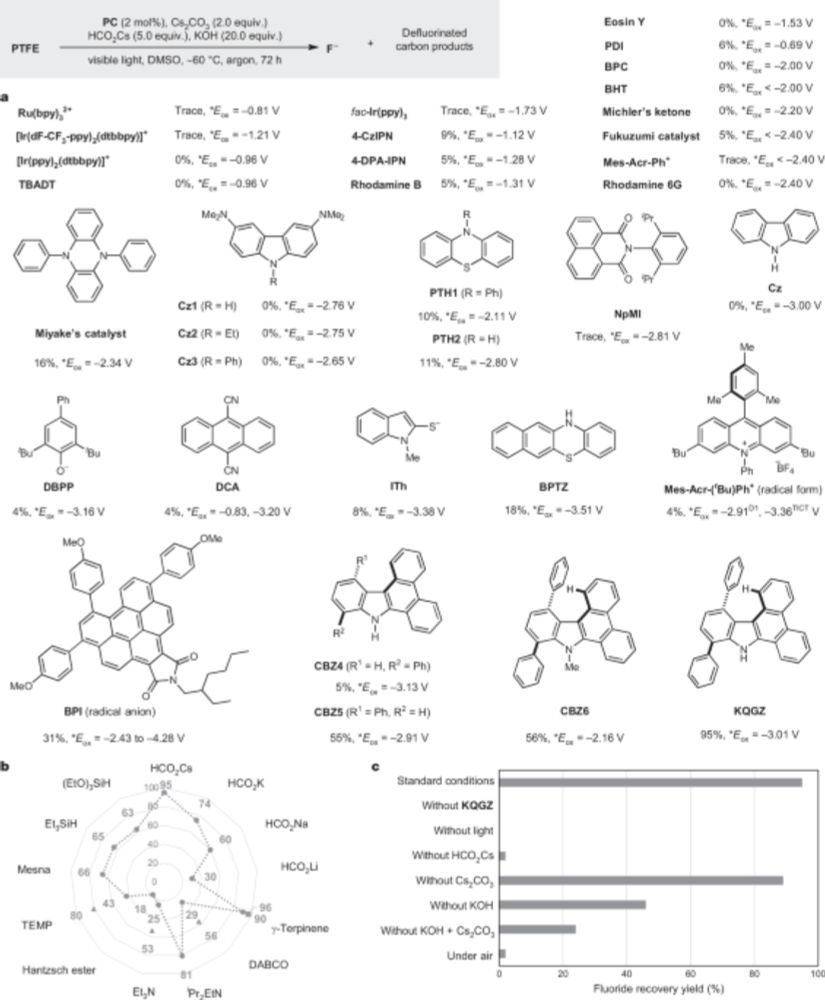
Photocatalytic low-temperature defluorination of PFASs - Nature
Photocatalysis at 40–60 °C is shown to be able to defluorinate perfluoroalkyl substances, known as ‘forever chemicals’, allowing the recycling of fluorine in polyfluoroalkyl and perfluoroalkyl substan...
www.nature.com
November 20, 2024 at 4:23 PM
There are two really cool #PFAS / #foreverchemicals degradation papers out in Nature today
In one, the authors show that powdered Teflon can be transformed into charcoal+fluoride with light+a catalyst+reducing reagent & some very important help from mainly KOH and Cs formate
This is bonkers cool:
In one, the authors show that powdered Teflon can be transformed into charcoal+fluoride with light+a catalyst+reducing reagent & some very important help from mainly KOH and Cs formate
This is bonkers cool:
Reposted by Megan R. Hill
Calling all polymer scientists! Let’s re-find each other as people continue to migrate over!
Please tag people in the comments and share! I’ll continue updating as we find more people. #polymers
go.bsky.app/AgjxzFa
Please tag people in the comments and share! I’ll continue updating as we find more people. #polymers
go.bsky.app/AgjxzFa
November 10, 2024 at 9:51 PM
Calling all polymer scientists! Let’s re-find each other as people continue to migrate over!
Please tag people in the comments and share! I’ll continue updating as we find more people. #polymers
go.bsky.app/AgjxzFa
Please tag people in the comments and share! I’ll continue updating as we find more people. #polymers
go.bsky.app/AgjxzFa

