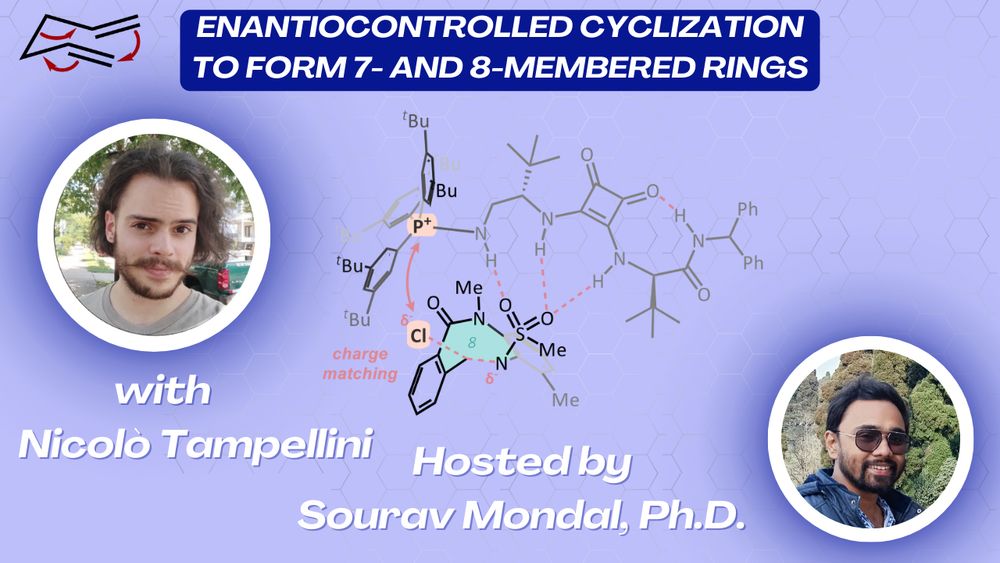
Asymmetric catalysis with flasks and computers ⚗️💻 https://ntampellini.github.io/

pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
g-xTB marks not just an evolution, but a revolution in the capabilities of semiempirical quantum chemistry. Convince yourself! A thread.
🔗 chemrxiv.org/engage/chemr...
#compchem

g-xTB marks not just an evolution, but a revolution in the capabilities of semiempirical quantum chemistry. Convince yourself! A thread.
🔗 chemrxiv.org/engage/chemr...
#compchem

doi.org/10.1016/j.ch...
doi.org/10.1016/j.ch...
Deadline: July 6
Link to our recent work in comments.

Deadline: July 6
Link to our recent work in comments.
it took a while, but now the ORCA 6.0 article is out! It serves as generic reference for ORCA 6.x. However, if you are serious about supporting our efforts, please take note of the suggested citations at the end of each ORCA run.
wires.onlinelibrary.wiley.com/doi/10.1002/...

it took a while, but now the ORCA 6.0 article is out! It serves as generic reference for ORCA 6.x. However, if you are serious about supporting our efforts, please take note of the suggested citations at the end of each ORCA run.
wires.onlinelibrary.wiley.com/doi/10.1002/...
Really important piece to share widely. Too many Americans don’t know how severe a threat this Administration poses to our scientific infrastructure, global leadership, and health security

Really important piece to share widely. Too many Americans don’t know how severe a threat this Administration poses to our scientific infrastructure, global leadership, and health security
Link: youtu.be/9e4Hxt5FjH4
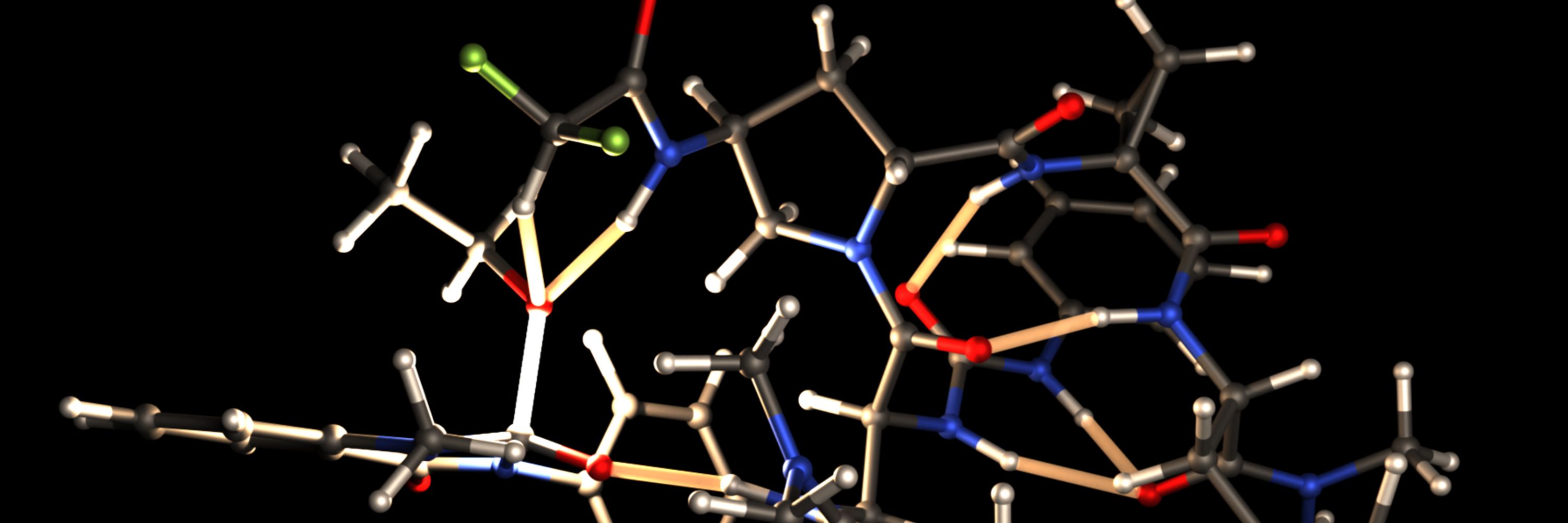
J. Am. Chem. Soc. 2025, 147, 4624-4630.
doi.org/10.1021/jacs...

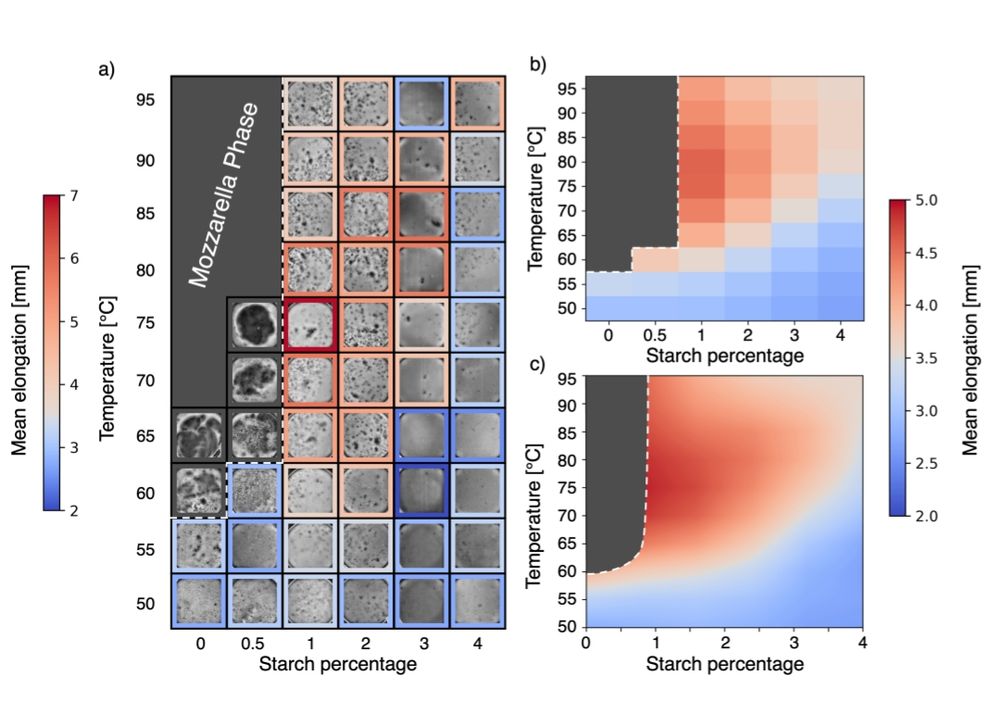
Prepare to get cheesy, I'm glad to share the Cacio e paper preprint:
arxiv.org/abs/2501.00536
Prepare to get cheesy, I'm glad to share the Cacio e paper preprint:
arxiv.org/abs/2501.00536
Our group is recruiting a postdoctoral researcher for a project on reactive machine learning potentials. Reposts and spreading the word to interested people in your network appreciated!
jobs.ethz.ch/job/view/JOP...



