
https://proteaglyco.com/
Short answer: trace amounts in human at best.
#glycotime

Short answer: trace amounts in human at best.
#glycotime
Using MS1, MS2, and LC, we reduced the number of putative identifications by 95%, ensuring the quality of these annotations.

Using MS1, MS2, and LC, we reduced the number of putative identifications by 95%, ensuring the quality of these annotations.
- O-acetylated structures elute later
- Their peak shape is broader
We're not quite sure why we have broader peak shapes, but it was universal. Further work is ongoing to resolve this, but it could be caused by multiple acetylation positions (more structures!).

- O-acetylated structures elute later
- Their peak shape is broader
We're not quite sure why we have broader peak shapes, but it was universal. Further work is ongoing to resolve this, but it could be caused by multiple acetylation positions (more structures!).
First fragmentation:
- Beam-type (HCD) CID is best, as it generates universally useful fragments for all acetylated structures
- Energy profiles show the expected ratios of these fragments

First fragmentation:
- Beam-type (HCD) CID is best, as it generates universally useful fragments for all acetylated structures
- Energy profiles show the expected ratios of these fragments
By performing native glycan analysis, we are suddenly seeing 2x the structures previously observed in rats and mice.
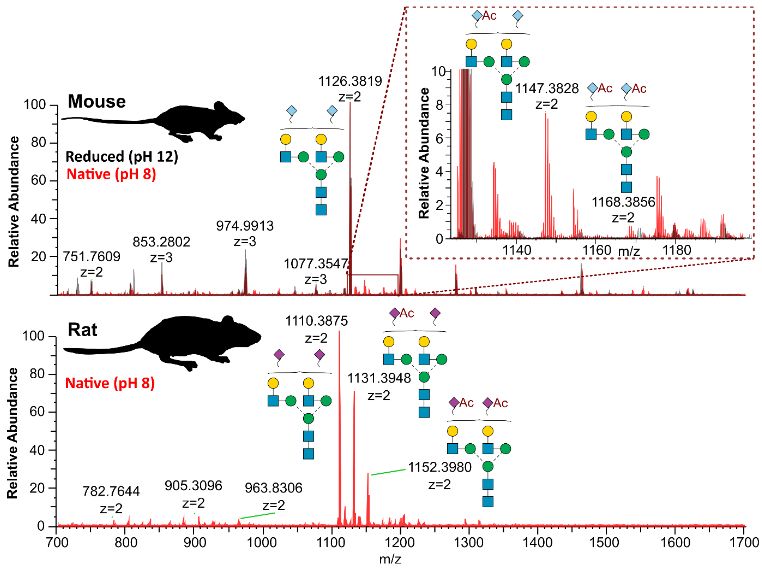
By performing native glycan analysis, we are suddenly seeing 2x the structures previously observed in rats and mice.

