The Guardian: www.theguardian.com/science/2026...
Reuters: www.reuters.com/business/hea...
The Times: www.thetimes.com/uk/science/a...
Boston Globe: www.bostonglobe.com/2026/01/09/n...
The Guardian: www.theguardian.com/science/2026...
Reuters: www.reuters.com/business/hea...
The Times: www.thetimes.com/uk/science/a...
Boston Globe: www.bostonglobe.com/2026/01/09/n...
This is, to our knowledge, the first molecular intervention shown to improve chromosome cohesion in human eggs.
(9/10)

This is, to our knowledge, the first molecular intervention shown to improve chromosome cohesion in human eggs.
(9/10)
This effect is consistent across donors. (8/10)

This effect is consistent across donors. (8/10)
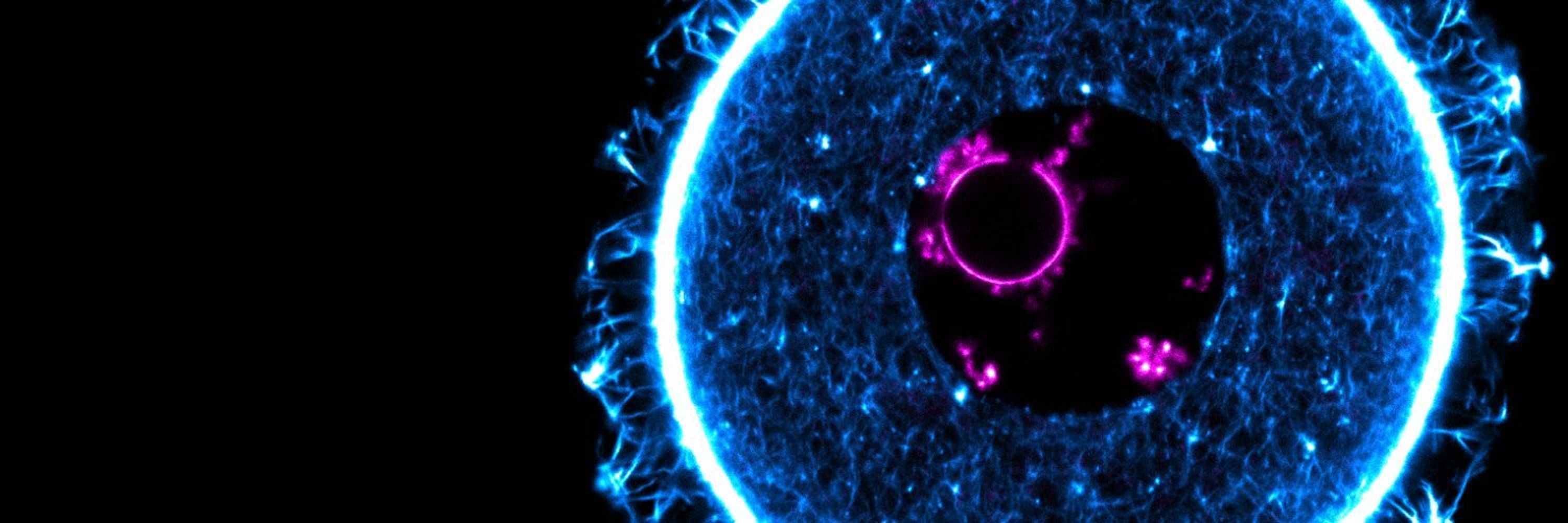
Eggs from women ≥35 years have significantly reduced SGO1 and increased PSSC compared to younger donors.
Can this be rescued by supplementing SGO1? (7/10)

Eggs from women ≥35 years have significantly reduced SGO1 and increased PSSC compared to younger donors.
Can this be rescued by supplementing SGO1? (7/10)

In aged mouse oocytes, we observe a decline in pericentromeric transcription, SGO1, PP2A, and centromeric cohesion.
This mirrors the dramatic rise in PSSC with age. (5/10)

In aged mouse oocytes, we observe a decline in pericentromeric transcription, SGO1, PP2A, and centromeric cohesion.
This mirrors the dramatic rise in PSSC with age. (5/10)
Blocking transcription reduces SGO1 on chromosomes and triggers cohesion loss. (4/10)

Blocking transcription reduces SGO1 on chromosomes and triggers cohesion loss. (4/10)
In mouse oocytes, depletion of SGO1 with siRNAs causes cohesion failure (PSSC) and alignment defects. But how is SGO1 maintained at meiotic centromeres? (3/10)

In mouse oocytes, depletion of SGO1 with siRNAs causes cohesion failure (PSSC) and alignment defects. But how is SGO1 maintained at meiotic centromeres? (3/10)
When cohesion fails, chromatids separate prematurely — a defect called PSSC (premature separation of sister chromatids) — leading to mis-segregation and aneuploidy. (2/10)

When cohesion fails, chromatids separate prematurely — a defect called PSSC (premature separation of sister chromatids) — leading to mis-segregation and aneuploidy. (2/10)
Thanks to @idajentoft.bsky.social for your great work on this review. (5/5)
Thanks to @idajentoft.bsky.social for your great work on this review. (5/5)

But how do oocytes store these proteins? (2/5)
But how do oocytes store these proteins? (2/5)

