Scott Gottlieb, MD
@scottgottliebmd.bsky.social
27K followers
1.7K following
150 posts
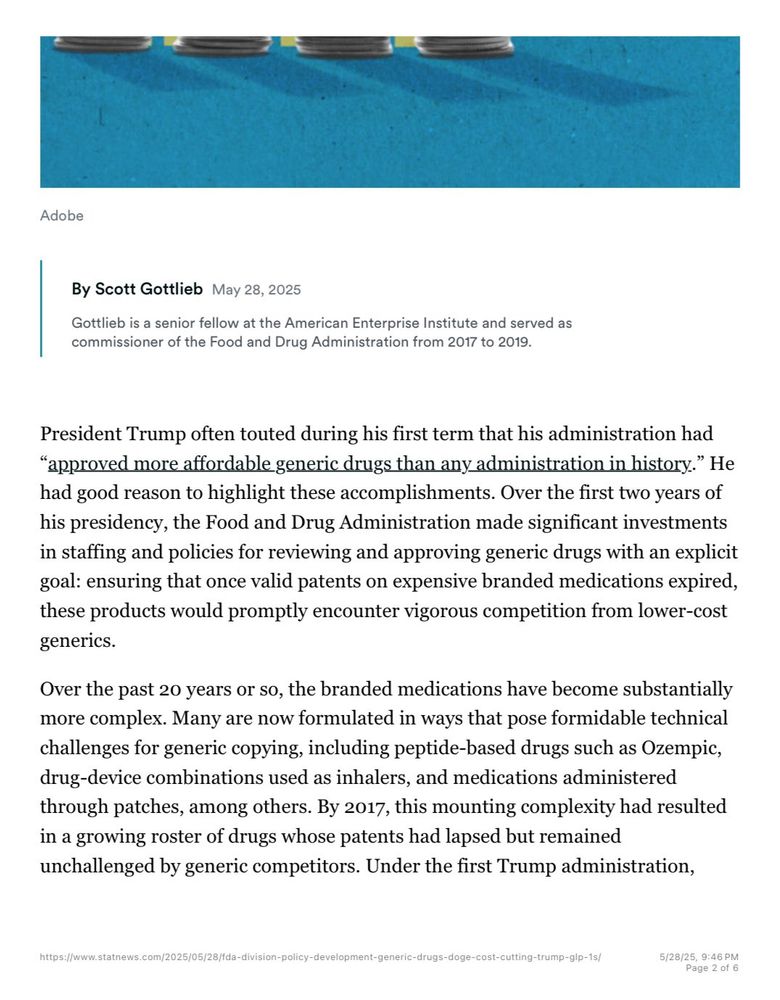
Senior Fellow at the American Enterprise Institute. Partner, New Enterprise Associates. Contributor CNBC. 23rd Commissioner of the U.S. FDA. Public boards: Pfizer, Illumina, TempusAI.
Posts
Media
Videos
Starter Packs
Reposted by Scott Gottlieb, MD
Reposted by Scott Gottlieb, MD
Reposted by Scott Gottlieb, MD
Reposted by Scott Gottlieb, MD
Reposted by Scott Gottlieb, MD
Reposted by Scott Gottlieb, MD
Reposted by Scott Gottlieb, MD