Sean Ong
@seanong.bsky.social
1K followers
170 following
86 posts
ID doc and joint PhD candidate at University of Toronto + University of Melbourne. Talk to me about clinical trial design and methodology, bloodstream infections, S. aureus, and Gram negatives 🦠
🇸🇬🇨🇦🇦🇺
Posts
Media
Videos
Starter Packs
Sean Ong
@seanong.bsky.social
· 10d
Sean Ong
@seanong.bsky.social
· 10d
Sean Ong
@seanong.bsky.social
· Jul 18
Sean Ong
@seanong.bsky.social
· Jul 18
Sean Ong
@seanong.bsky.social
· Jun 26
Evaluating the impact of a SIMPlified LaYered consent process on recruitment of potential participants to the Staphylococcus aureus Network Adaptive Platform trial: study protocol for a multicentre pr...
Introduction Informed consent forms (ICFs) for randomised clinical trials (RCTs) can be onerous and lengthy. The process has the potential to overwhelm patients with information, leading them to miss ...
doi.org
Sean Ong
@seanong.bsky.social
· Jun 26

Trials Within Trials—Optimizing the Delivery of RCTs
In the latest issue of JAMA, Johansen and colleagues1 report the results of a trial that investigated the impact of digital recruitment letter formats on recruitment to a larger clinical trial, the DA...
jamanetwork.com
Sean Ong
@seanong.bsky.social
· Jun 16
Sean Ong
@seanong.bsky.social
· Jun 16
Sean Ong
@seanong.bsky.social
· Jun 16

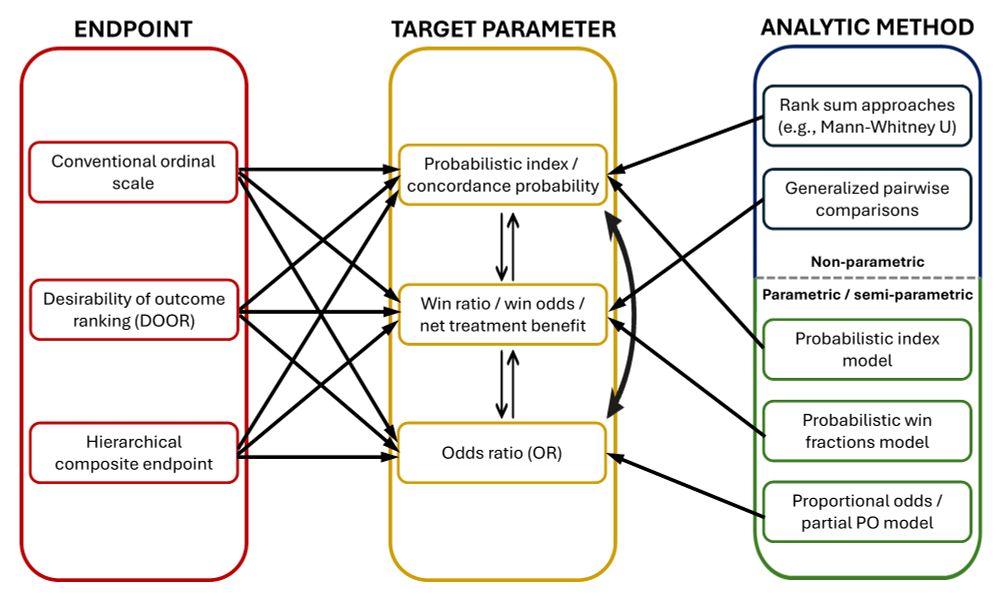
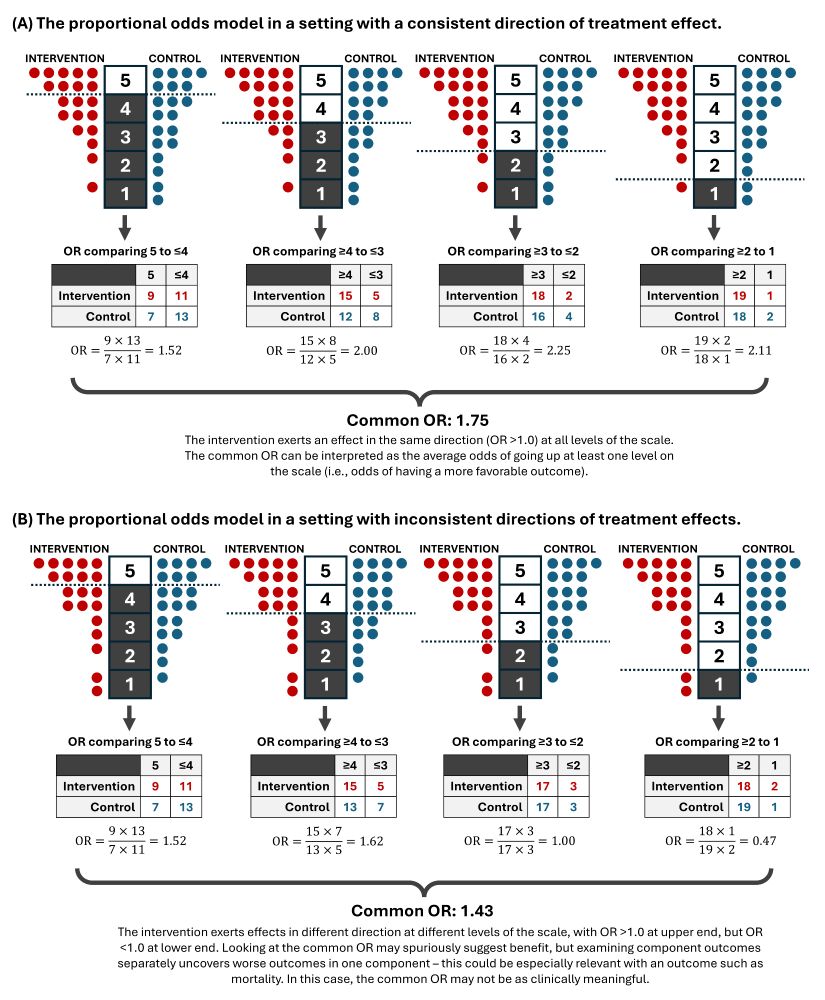
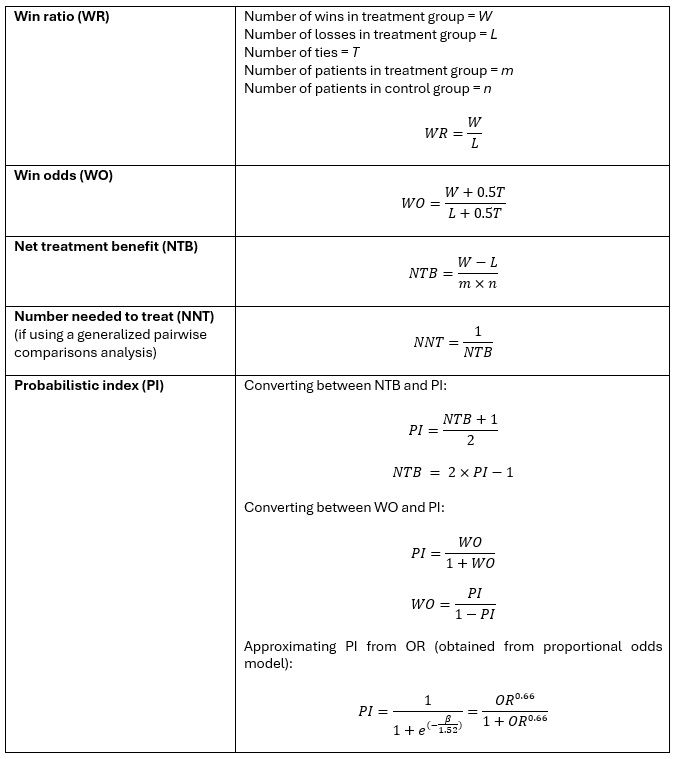
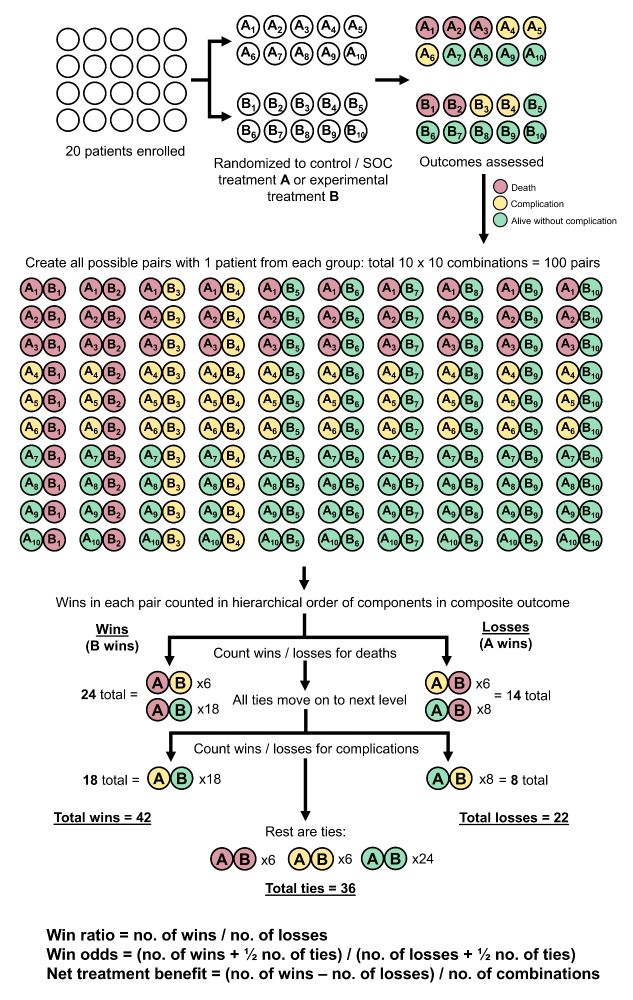
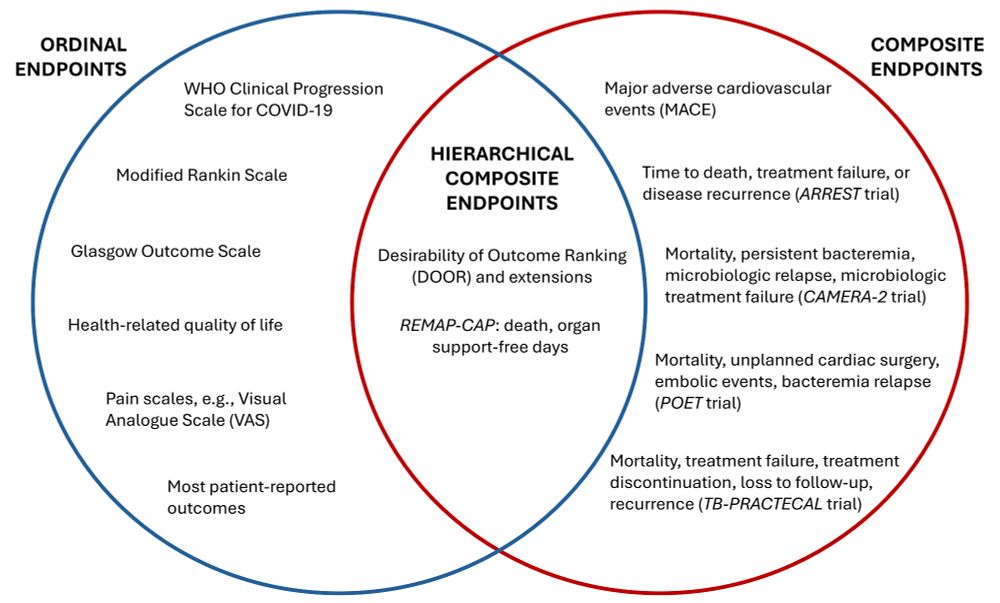
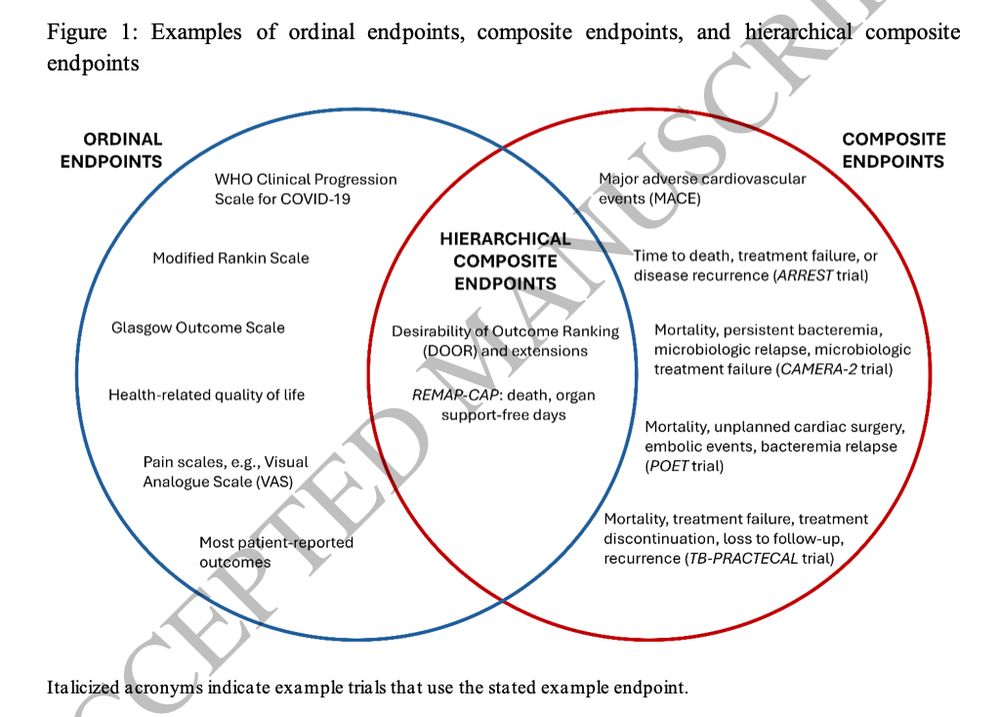
Making sense of hierarchical composite endpoints in randomized clinical trials – a primer for infectious disease clinicians and researchers
Hierarchical composite endpoints (HCEs) are increasingly being used in infectious diseases research. In this review aimed at educating the clinical reader,
doi.org
Reposted by Sean Ong
Sean Ong
@seanong.bsky.social
· May 20

Target Trial Emulation for Antibiotic Use in Acute COVID-19
This study by Pulia and colleagues1 reports results from a large population-based cohort using target trial emulation to examine the associations of antibiotic treatment with clinical outcomes in pati...
jamanetwork.com
Reposted by Sean Ong
Reposted by Sean Ong
Sean Ong
@seanong.bsky.social
· May 10
Sean Ong
@seanong.bsky.social
· May 7