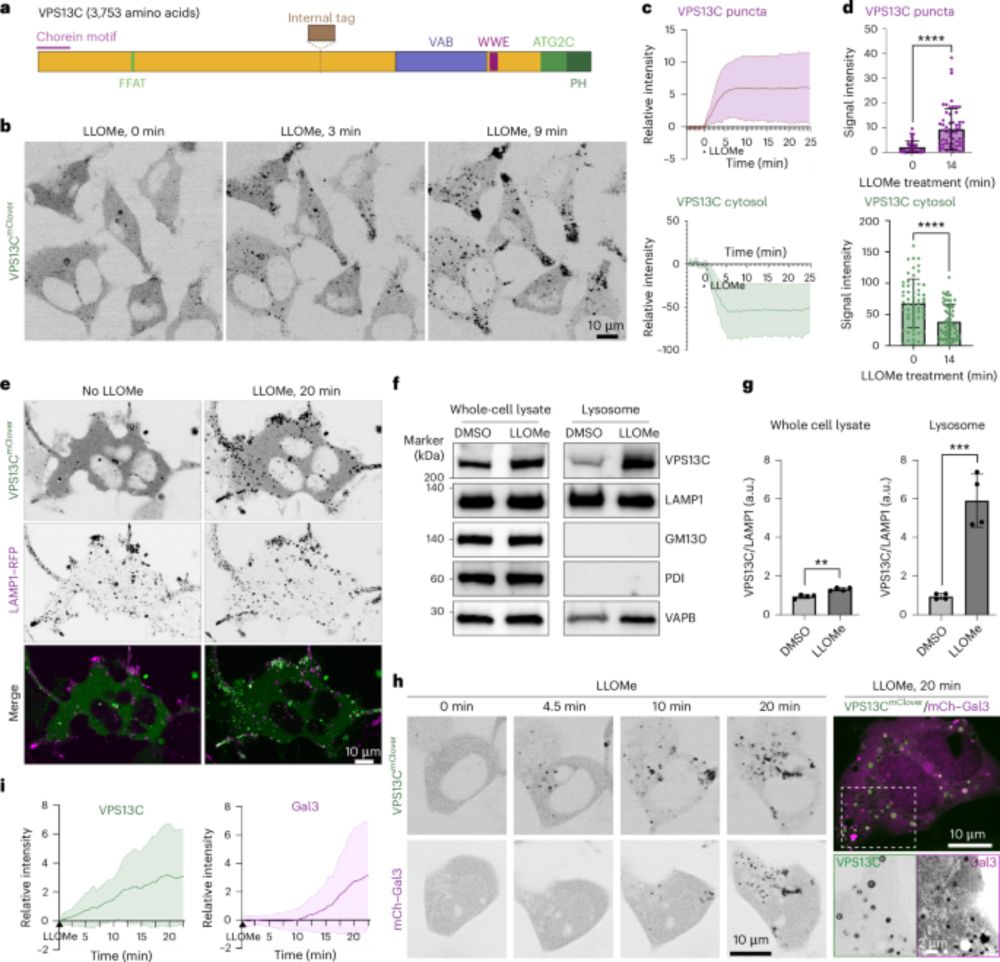
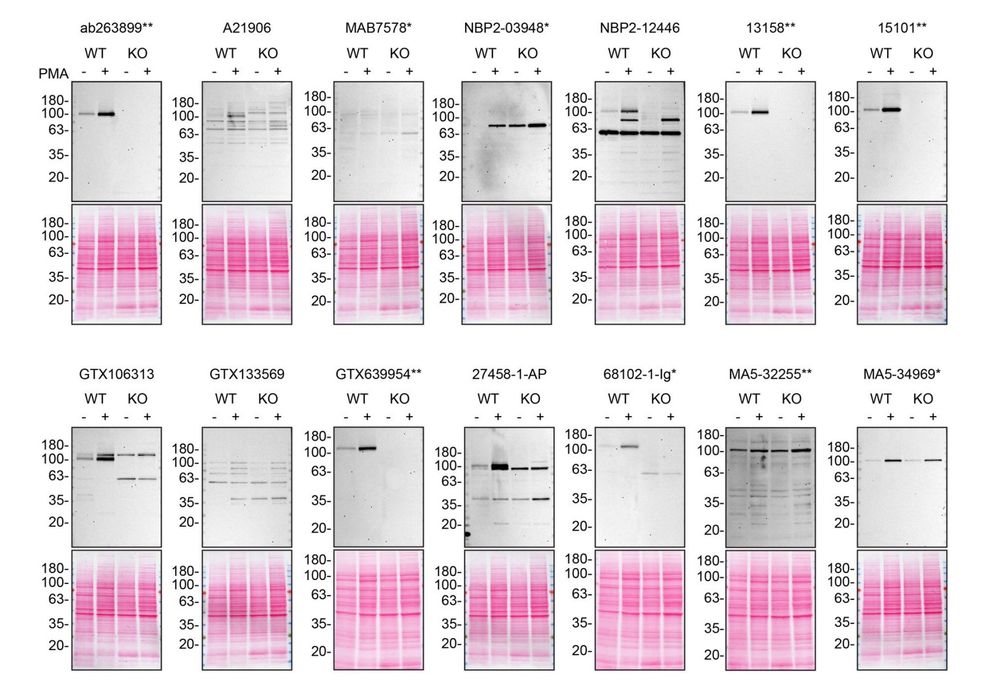
shawnferguson.bsky.social
@shawnferguson.bsky.social
1.1K followers
470 following
110 posts
Cell Biologist and Neuroscientist at Yale University. Outdoor explorer.
Posts
Media
Videos
Starter Packs
Reposted by shawnferguson.bsky.social
Reposted by shawnferguson.bsky.social
Reposted by shawnferguson.bsky.social
Reposted by shawnferguson.bsky.social
Reposted by shawnferguson.bsky.social
Reposted by shawnferguson.bsky.social
Reposted by shawnferguson.bsky.social