
sushilong.netlify.app
pubs.acs.org/doi/full/10....
Let’s make chemistry constantly reproducible!
#Reproducibility #Replicability #Chemistry #Catalysis #Nanoparticles
@acs.org @pubs.acs.org @jacs.acspublications.org

pubs.acs.org/doi/full/10....
Let’s make chemistry constantly reproducible!
#Reproducibility #Replicability #Chemistry #Catalysis #Nanoparticles
@acs.org @pubs.acs.org @jacs.acspublications.org
Read it for free here: pubs.rsc.org/doi/D5S...

Read it for free here: pubs.rsc.org/doi/D5S...
Read it for free here: pubs.rsc.org/doi/D5S...

Read it for free here: pubs.rsc.org/doi/D5S...

With @philippseitz.bsky.social, @uniulm.bsky.social

With @philippseitz.bsky.social, @uniulm.bsky.social
Article by Yan Li & co-workers
Catalytic Joule heating synthesis of one-dimensional nanomaterials in seconds
www.nature.com/articles/s44... ($)
#Chemsky

Article by Yan Li & co-workers
Catalytic Joule heating synthesis of one-dimensional nanomaterials in seconds
www.nature.com/articles/s44... ($)
#Chemsky

Article by Yang Zhang, Zhan’ao Tan, Fanglong Yuan, Louzhen Fan & co-workers
Pure-violet oxygen-doped carbon quantum rings with near-unity quantum yield and a full-width at half-maximum of 18 nm
www.nature.com/articles/s44... ($)
#Chemsky

Article by Yang Zhang, Zhan’ao Tan, Fanglong Yuan, Louzhen Fan & co-workers
Pure-violet oxygen-doped carbon quantum rings with near-unity quantum yield and a full-width at half-maximum of 18 nm
www.nature.com/articles/s44... ($)
#Chemsky
Article by Yang Zhang, Zhan’ao Tan, Fanglong Yuan, Louzhen Fan & co-workers
Pure-violet oxygen-doped carbon quantum rings with near-unity quantum yield and a full-width at half-maximum of 18 nm
www.nature.com/articles/s44... ($)
#Chemsky

Article by Zebing Zeng, Jishan Wu, Yong Ni & co-workers
Aromaticity and through-space electronic coupling in [2]catenanes comprising two intertwined globally electron-delocalized octaphyrinoid rings
www.nature.com/articles/s44... ($)
#Chemsky


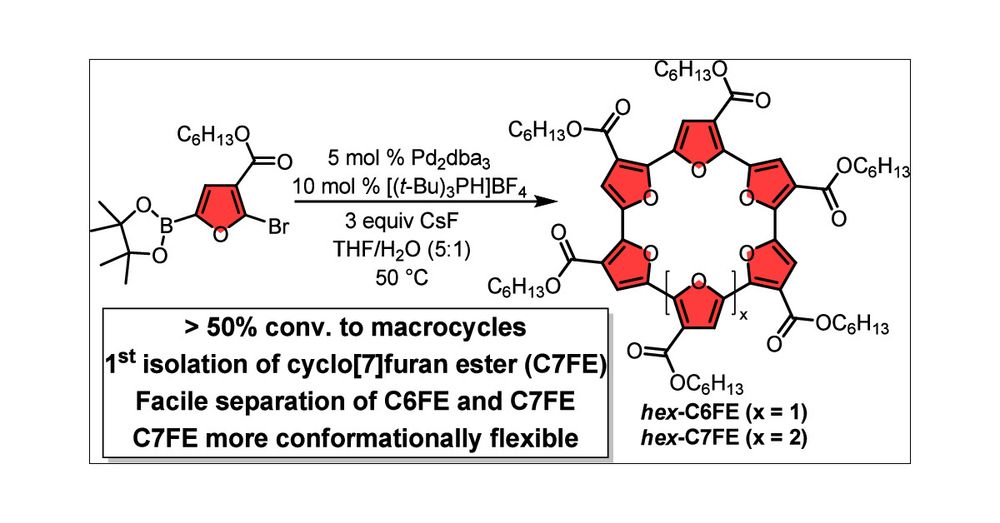
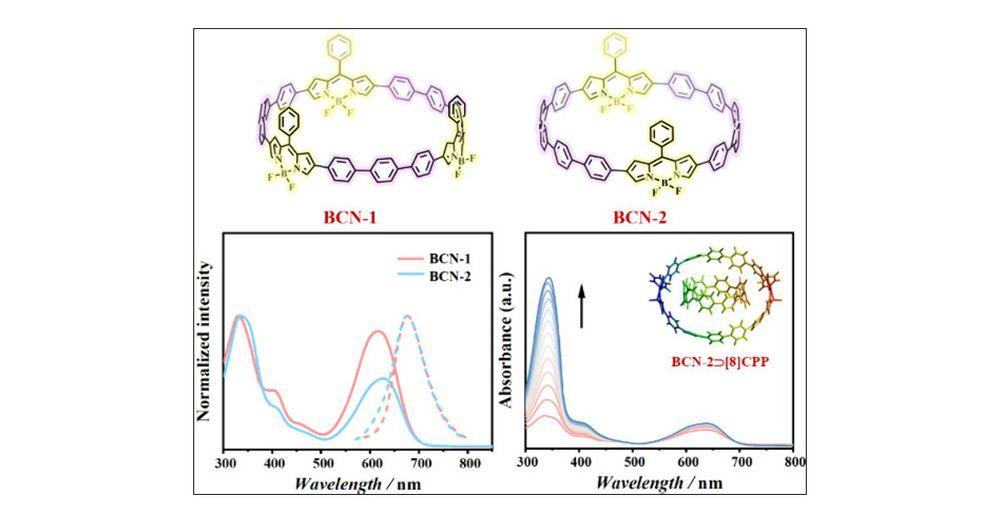
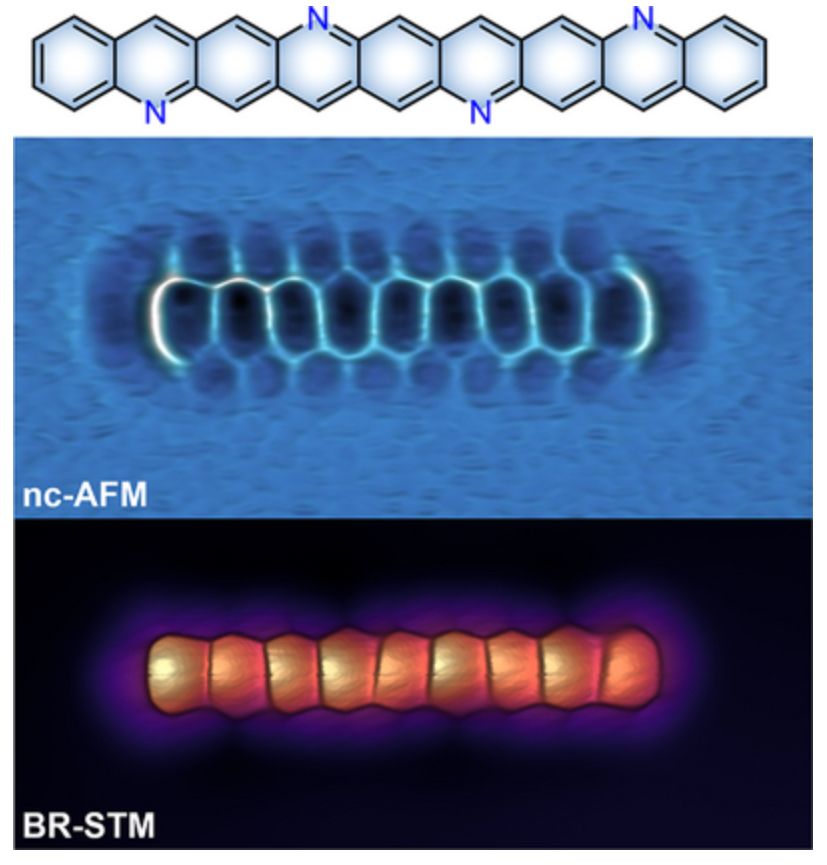








pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...



