Tehshik Yoon
@tehshik.bsky.social
2K followers
460 following
120 posts
Professor of Chemistry, University of Wisconsin-Madison. Associate Editor, ACS Catalysis. Big ol nerd, all contexts. 🇺🇸🇰🇷🇨🇦🏳️🌈 (he/him/his)
Posts
Media
Videos
Starter Packs
Reposted by Tehshik Yoon
Mark Histed
@markhisted.org
· 16d
Reposted by Tehshik Yoon
Jeff Martell
@jeffmartell.bsky.social
· Aug 14
DNA-Scaffolded Ultrahigh-Throughput Reaction Screening
Discovering and optimizing reactions is central to synthetic chemistry. However, chemical reactions are traditionally screened using relatively low-throughput methods, prohibiting exploration of diver...
chemrxiv.org
Tehshik Yoon
@tehshik.bsky.social
· Jul 30
Reposted by Tehshik Yoon
Reposted by Tehshik Yoon
Reposted by Tehshik Yoon
Tehshik Yoon
@tehshik.bsky.social
· Apr 29
Reposted by Tehshik Yoon
Tehshik Yoon
@tehshik.bsky.social
· Mar 19
Reposted by Tehshik Yoon
Bach Lab | TUM
@bach-lab.bsky.social
· Mar 14
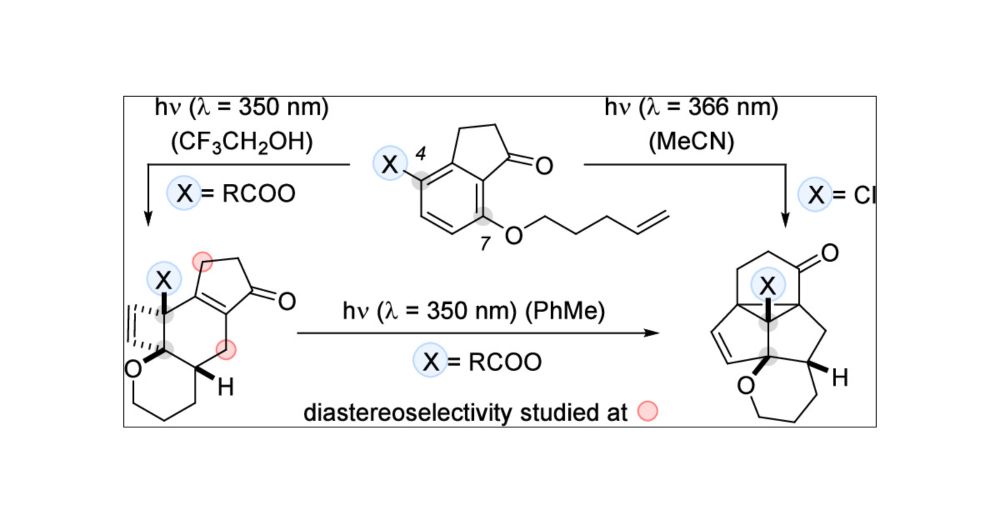
Intramolecular ortho Photocycloaddition of 4-Substituted 7-(4′-Alkenyloxy)-1-indanones and Ensuing Reaction Cascades
4-Substituted 7-(4′-alkenyloxy)-1-indanones were prepared from the respective substituted aryl propanoic acids and subjected to UV-A irradiation (λ = 350 or 366 nm). While the 4-chloro compound was directly converted at λ = 366 nm into a pentacyclic product (47% yield) by a three-photon cascade process, the oxygenated substrates reacted in trifluoroethanol at λ = 350 nm by a two-photon cascade, involving an ortho photocycloaddition, a thermal disrotatory ring opening, and a [4π] photocyclization (six examples, 67–82% yield). An ensuing photochemical di-π-methane rearrangement of the latter products was achieved by irradiation at λ = 350 nm in toluene (five examples, 36–70% yield). The diastereoselectivity of the reaction was probed employing a chiral 1-indanone with a stereogenic center at carbon atom C3. 1-Indanones with a 4-hexenyloxy side chain [(E)- or (Z)-configured] at carbon atom C7 served to interrogate the stereospecifity of the reaction.
pubs.acs.org
Tehshik Yoon
@tehshik.bsky.social
· Mar 8
Tehshik Yoon
@tehshik.bsky.social
· Feb 28
Reposted by Tehshik Yoon
Reposted by Tehshik Yoon
Bach Lab | TUM
@bach-lab.bsky.social
· Feb 25
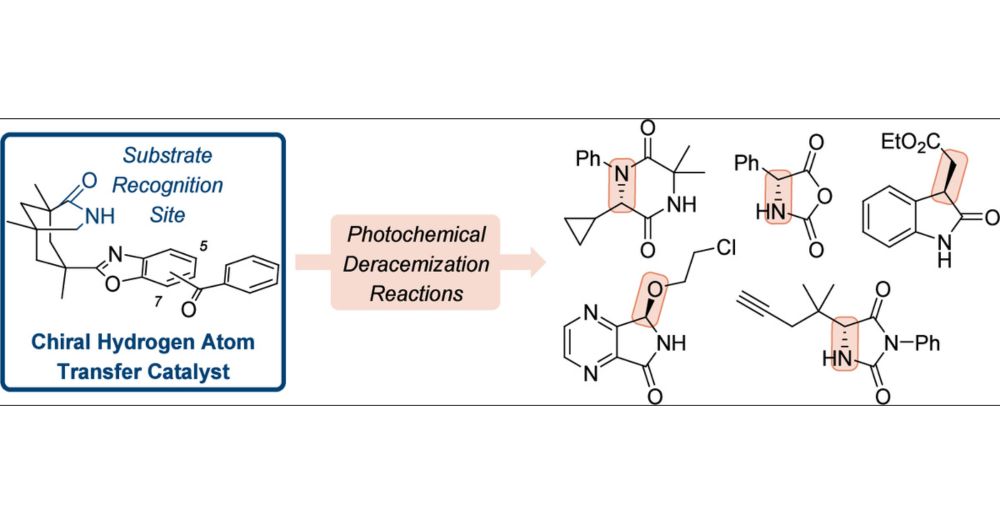
Stereochemical Editing at sp3-Hybridized Carbon Centers by Reversible, Photochemically Triggered Hydrogen Atom Transfer
ConspectusMillions of chiral compounds contain a stereogenic sp3-hybridized carbon center with a hydrogen atom as one of the four different substituents. The stereogenic center can be edited in an increasing number of cases by selective hydrogen atom transfer (HAT) to and from a photocatalyst. This Account describes the development of photochemical deracemization reactions using chiral oxazole-annulated benzophenones with a bonding motif that allows them to recognize chiral lactam substrates by two-point hydrogen bonding. The backbone of the catalysts consists of a chiral azabicyclo[3.3.1]nonan-2-one with a U-shaped geometry, which enables substrate recognition to occur parallel to the benzoxazole part of the aromatic ketones. The photocatalysts facilitate a catalytic photochemical deracemization of several compound classes including hydantoins, N-carboxyanhydrides, oxindoles, 2,5-diketopiperazines, and 4,7-diaza-1-isoindolinones. In addition, if more than one stereogenic center is present, the editing delivers a distinct diastereoisomer upon the appropriate selection of the respective photocatalyst enantiomer. The chiral photocatalysts operate via the benzophenone triplet that selectively abstracts a properly positioned hydrogen atom in exclusively one of the two substrate enantiomers. The photochemical step creates a planar carbon-centered radical and erases the absolute configuration at this position. While returning HAT to the same position would likely recreate the stereogenic center with the same absolute configuration, spectroscopic and quantum chemical studies suggest that the hydrogen atom is delivered from the photocatalyst to a heteroatom that is in conjugation to the radical center. Two scenarios can be distinguished for the hydrogen atom shuttling process. For hydantoins, N-carboxyanhydrides, and 4,7-diaza-1-isoindolinones, the back HAT occurs to a carbonyl oxygen atom or an imine-type nitrogen atom which is not involved in binding to the catalyst. For oxindoles and 2,5-diketopiperazines, a single lactam carbonyl group in the substrate is available to accept the hydrogen atom. It is currently assumed that back HAT occurs to this group, although the carbonyl oxygen atom is involved in hydrogen bonding to the catalyst. In comparison to the former reaction pathway, the latter process appears to be less efficient and more prone to side reactions. For both cases, an achiral enol or enamine is formed, which delivers upon dissociation from the catalyst statistically either one of the two stereoisomers of the substrate. Since only one substrate enantiomer (or diastereoisomer) is processed, a high enantioselectivity (or diastereoselectivity) results. Even though the editing is a contra-thermodynamic process, the described decoupling of a photochemical and a thermal step allows the usage of a single catalyst in loadings that vary between 2.5 and 10 mol % depending on the specific mode of action.
pubs.acs.org
Reposted by Tehshik Yoon
Tehshik Yoon
@tehshik.bsky.social
· Jan 29
Reposted by Tehshik Yoon