Tianxiong (Bear) Yu
@tianxiong-bear-yu.bsky.social
78 followers
150 following
14 posts
Instructor in UMass Chan Medical School. Focus on using Computational Methods to study transposons in human and koala genomes.
Posts
Media
Videos
Starter Packs
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
Selin Jessa
@selinjessa.bsky.social
· May 3

Dissecting regulatory syntax in human development with scalable multiomics and deep learning
Transcription factors (TFs) establish cell identity during development by binding regulatory DNA in a sequence-specific manner, often promoting local chromatin accessibility, and regulating gene expre...
www.biorxiv.org
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
Johan Jakobsson
@jakobssonlab.bsky.social
· Mar 29

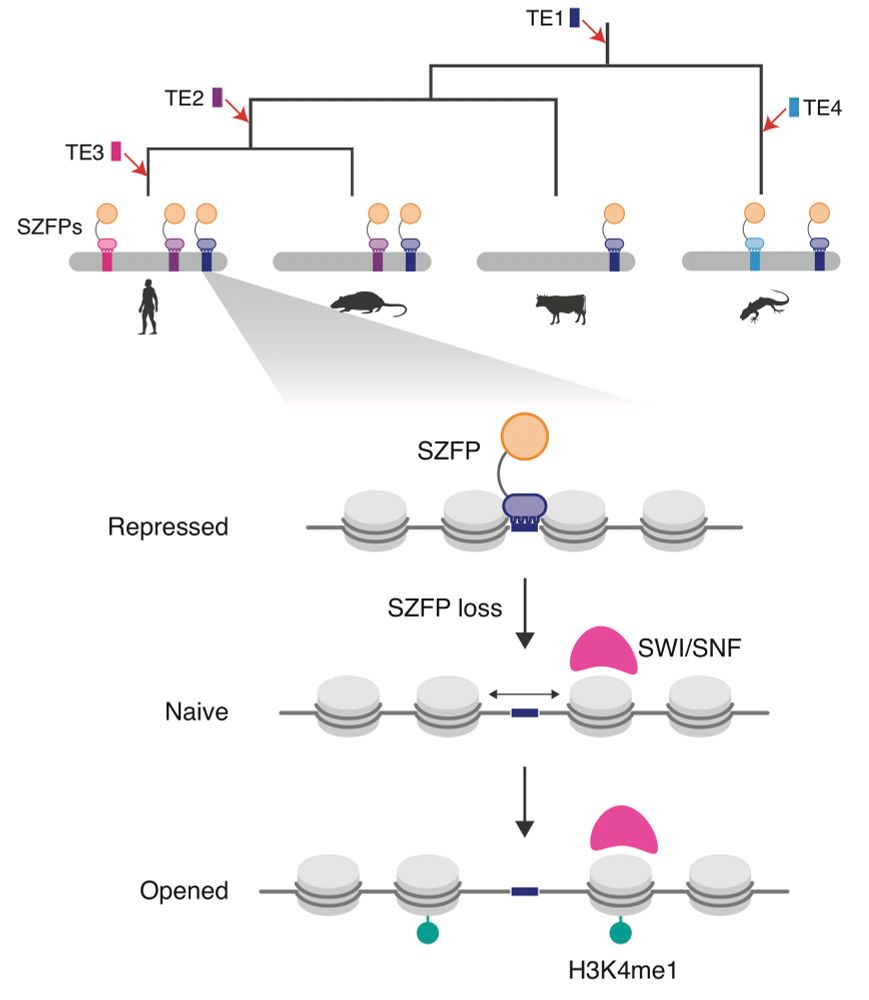
Loss of H3K9me3 maintenance in human neural progenitor cells leads to transcriptional activation of L1 retrotransposons
Heterochromatin is characterised by an inaccessibility to the transcriptional machinery and associated with the histone mark H3K9me3. Heterochromatin erosion is a hallmark of human ageing and H3K9me3 ...
www.biorxiv.org
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
@LaBonneLaB
@labonnelab.bsky.social
· Mar 19

Repurposing of a gill gene regulatory program for outer-ear evolution - Nature
A study on the evolutionary origin of the mammalian outer ear finds that it shares genetic programs with the gills of fishes and amphibians, indicating that elements of an ancestral gill have been reu...
www.nature.com
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu
Reposted by Tianxiong (Bear) Yu