Tobias Raisch
@traisch.bsky.social
160 followers
180 following
27 posts
Biochemist and Structural Biologist | Project group leader MPI Dortmund | He/him | Vegan bike punk #LeaveNoOneBehind
Posts
Media
Videos
Starter Packs
Reposted by Tobias Raisch
Nature
@nature.com
· 18d

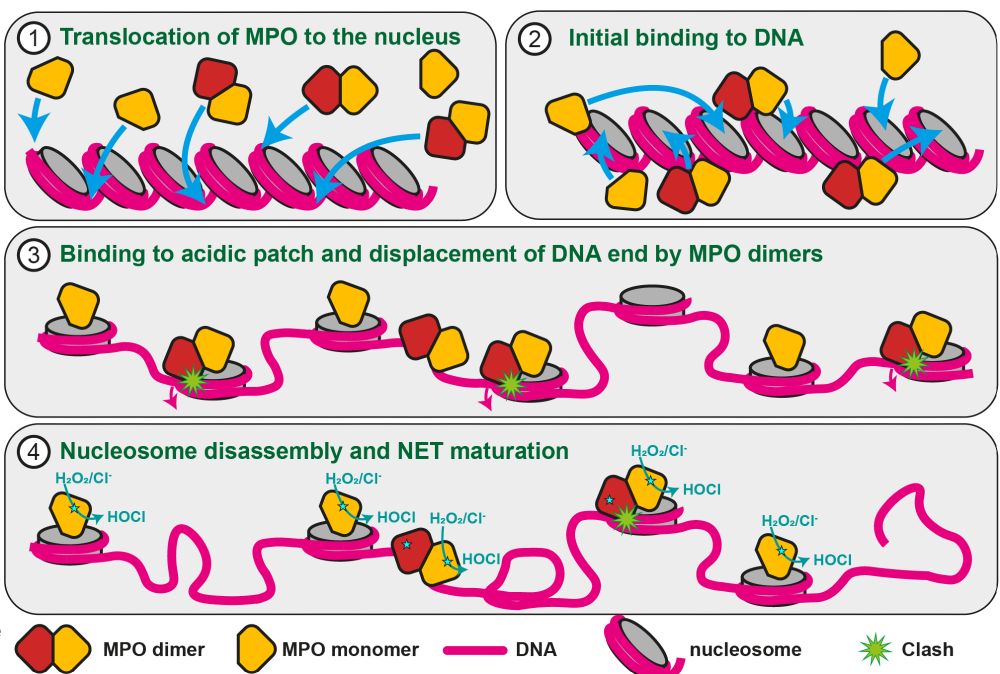
Myeloperoxidase transforms chromatin into neutrophil extracellular traps - Nature
Myeloperoxidase, a highly expressed neutrophil protein, disassembles nucleosomes, facilitating neutrophil extracellular trap (NET) formation, and binds stably to NETs extracellularly.
go.nature.com
Tobias Raisch
@traisch.bsky.social
· Jul 1
Tobias Raisch
@traisch.bsky.social
· Nov 21
Tobias Raisch
@traisch.bsky.social
· Nov 4
Tobias Raisch
@traisch.bsky.social
· Nov 4