Varun Gadkari
@varun-gadkari.bsky.social
120 followers
150 following
12 posts
Assistant Professor | Department of Chemistry, University of Minnesota | Biological Mass Spectrometry | Views are my own | http://gadkari.chem.umn.edu
Posts
Media
Videos
Starter Packs
Reposted by Varun Gadkari
The Associated Press
@apnews.com
· Apr 21

Harvard sues Trump administration to stop the freeze of more than $2 billion in grants
Harvard University has announced that it's suing the Trump administration to halt a freeze on more than $2.2 billion in grants after the institution said it would defy the Trump administration’s demands to limit activism on campus.
bit.ly
Reposted by Varun Gadkari
Reposted by Varun Gadkari
Ian Webb
@profianwebb.bsky.social
· Apr 11

Strategies for Acid and Amine Cross-linking and Labeling for Protein Structural Characterization Using Mass Spectrometry
Cross-linking and covalent labeling are common tools for protein structural elucidation by mass spectrometry. However, despite the importance of electrostatic interactions in proteins, there are not m...
pubs.acs.org
Reposted by Varun Gadkari
Reposted by Varun Gadkari
Reposted by Varun Gadkari
MaggieBeth 🪐🔭
@maggiebeth.space
· Apr 8
Reposted by Varun Gadkari
Reposted by Varun Gadkari
Jeremy Berg
@jeremymberg.bsky.social
· Apr 2
Reposted by Varun Gadkari
grimmrad 🧬🎼🖼️🏛️
@grimmrad.bsky.social
· Apr 1
Reposted by Varun Gadkari
grimmrad 🧬🎼🖼️🏛️
@grimmrad.bsky.social
· Apr 1
Reposted by Varun Gadkari
Kermit Murray
@kkmurray.bsky.social
· Apr 2

Native Top-Down Proteomics of Endogenous Protein Complexes Enabled by Online Two-Dimensional Liquid Chromatography
Protein complexes are essential for virtually all biological processes, yet their structural characterization remains a major challenge due to their heterogeneous, dynamic nature and the complexity of the proteome. Native top-down mass spectrometry (nTDMS) has emerged as a powerful tool for comprehensive structural characterization of purified protein complexes, but its application to endogenous protein complexes in the proteome is challenging and typically requires labor-intensive and time-consuming prefractionation. Here, for the first time, we develop a nondenaturing online two-dimensional liquid chromatography (2D-LC) method for native top-down proteomics (nTDP), enabling high-throughput structural analysis of endogenous protein complexes. The automated, online interfacing of size-exclusion and mixed-bed ion-exchange chromatography achieves high coverage of endogenous protein complexes. We further develop a multistage nTDMS approach that enables comprehensive structural characterization within the chromatographic timescale, capturing intact non-covalent complexes, released subunits/cofactors, and backbone fragments. Our analysis detected 133 native proteoforms and endogenous protein complexes (up to 350 kDa) from human heart tissue in less than two hours. Such technological leaps in high-throughput structural characterization of endogenous protein complexes will advance large-scale nTDP studies in health and disease.
dlvr.it
Reposted by Varun Gadkari
Reposted by Varun Gadkari
Chouinard Lab
@chouinardlab.bsky.social
· Mar 19
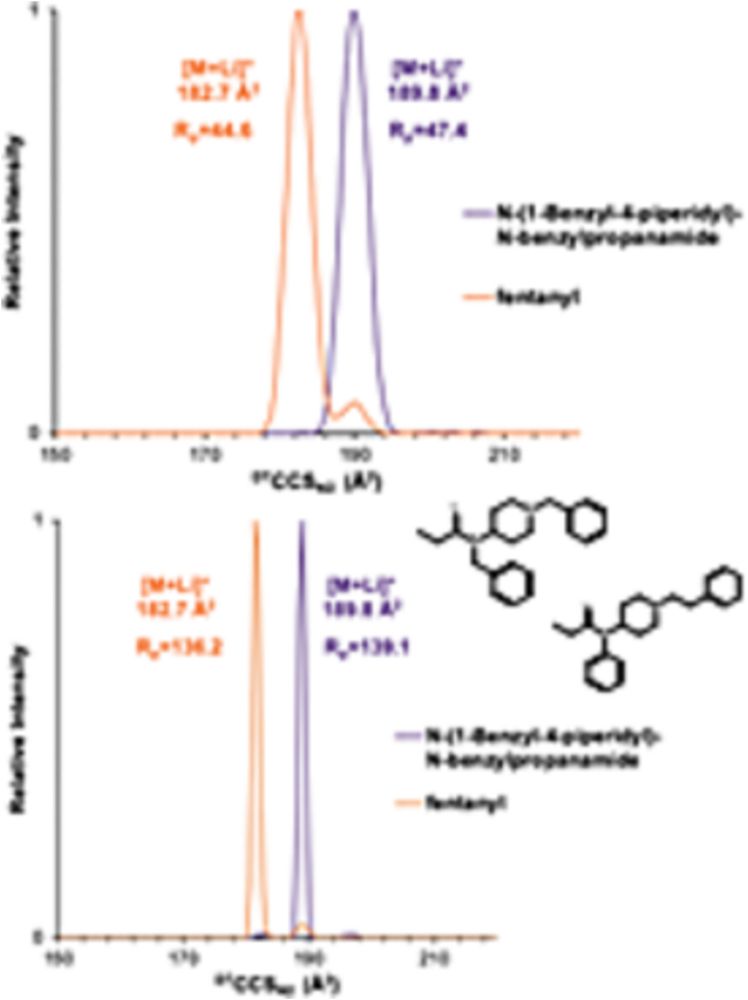
Improved separation of fentanyl isomers using metal cation adducts and high‐resolution ion mobility‐mass spectrometry
Fentanyl has become a growing concern as a deadly drug of abuse. In this work, we use a novel ion mobility–mass spectrometry (IM-MS) approach with metal cation adducts and high-resolution demultiplex...
analyticalsciencejournals.onlinelibrary.wiley.com
Reposted by Varun Gadkari
Chouinard Lab
@chouinardlab.bsky.social
· Mar 17
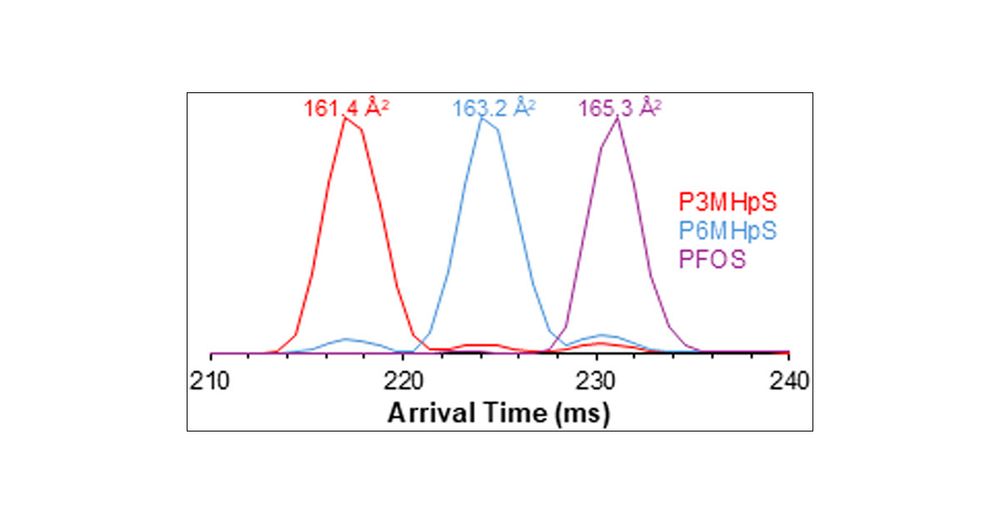
Multidimensional Separations for Characterization of Isomeric PFAS Using SLIM High-Resolution Ion Mobility and Tandem Mass Spectrometry
Per- and polyfluoroalkyl substances (PFAS) are synthetic organofluorine compounds that accumulate in the environment due to significant industrial use and resistance to degradation. PFAS are of global interest because of their environmental and health concerns. They exist in a variety of linear and nonlinear forms containing a variety of isomers, as well as differing functional headgroups for each class. That structural complexity requires advanced analytical techniques, beyond current high-resolution mass spectrometry (HRMS) methods, for their accurate identification and quantification in a wide range of samples. Herein, we demonstrate the power of Structures for Lossless Ion Manipulations (SLIM)-based high-resolution ion mobility (HRIM) for separation of complex PFAS branched isomers. SLIM is integrated into a multidimensional LC-SLIM IM-MS/MS workflow, developed for the extensive characterization of a wide range of PFAS compounds. As we surveyed sulfonate and carboxylic acid classes of PFAS, we observed unique arrival time vs m/z trend lines that were representative of each class; these trend lines are important for allowing identification of emerging species based on their placement in that two-dimensional space. Next, we used complementary tandem mass spectrometry (MS/MS) approaches with all ion fragmentation (AIF), as well as energy-resolved MS/MS, to further investigate the structure of mobility-separated species. This allowed both investigation of fragmentation mechanism and identification of unique fragment ions that could allow differentiation of isomers when ion mobility was insufficient. Overall, the combination of chromatography, high-resolution SLIM, and MS/MS provided a comprehensive workflow capable of identifying unknown emerging PFAS compounds in complex environmental samples.
pubs.acs.org