You know, for my menopause hot flashes!
Quite a stupid world we’ve built here.
You know, for my menopause hot flashes!
Quite a stupid world we’ve built here.
Elinzanetant is a neurokinin 1 and neurokinin 3 receptor antagonist for M/S vasomotor symptoms due to menopause.
Riboflavin 5’-phosphate is a corneal cross-linking …
Continued
2/3

Elinzanetant is a neurokinin 1 and neurokinin 3 receptor antagonist for M/S vasomotor symptoms due to menopause.
Riboflavin 5’-phosphate is a corneal cross-linking …
Continued
2/3
A. Elinzanetant (Lynkuet)
B. Riboflavin 5’-phosphate (Epioxa)
C. Suzetrigine (Journavx)
D. Vimseltinib (Romvimza)
The answer is in the thread 1/3
#PhunQuiz

A. Elinzanetant (Lynkuet)
B. Riboflavin 5’-phosphate (Epioxa)
C. Suzetrigine (Journavx)
D. Vimseltinib (Romvimza)
The answer is in the thread 1/3
#PhunQuiz
Free access: medicalletter.org/elinz744b

Free access: medicalletter.org/elinz744b
Full video: www.contemporaryobgyn.net/view/genevie...
Full video: www.contemporaryobgyn.net/view/genevie...
🔗 Full article: stellanews.life/technology_c...

🔗 Full article: stellanews.life/technology_c...
#Menopause #BreastCancer #HotFlashes

#Menopause #BreastCancer #HotFlashes
Check out the full case study on Drug Hunter: drughunter.com/molecule/eli...
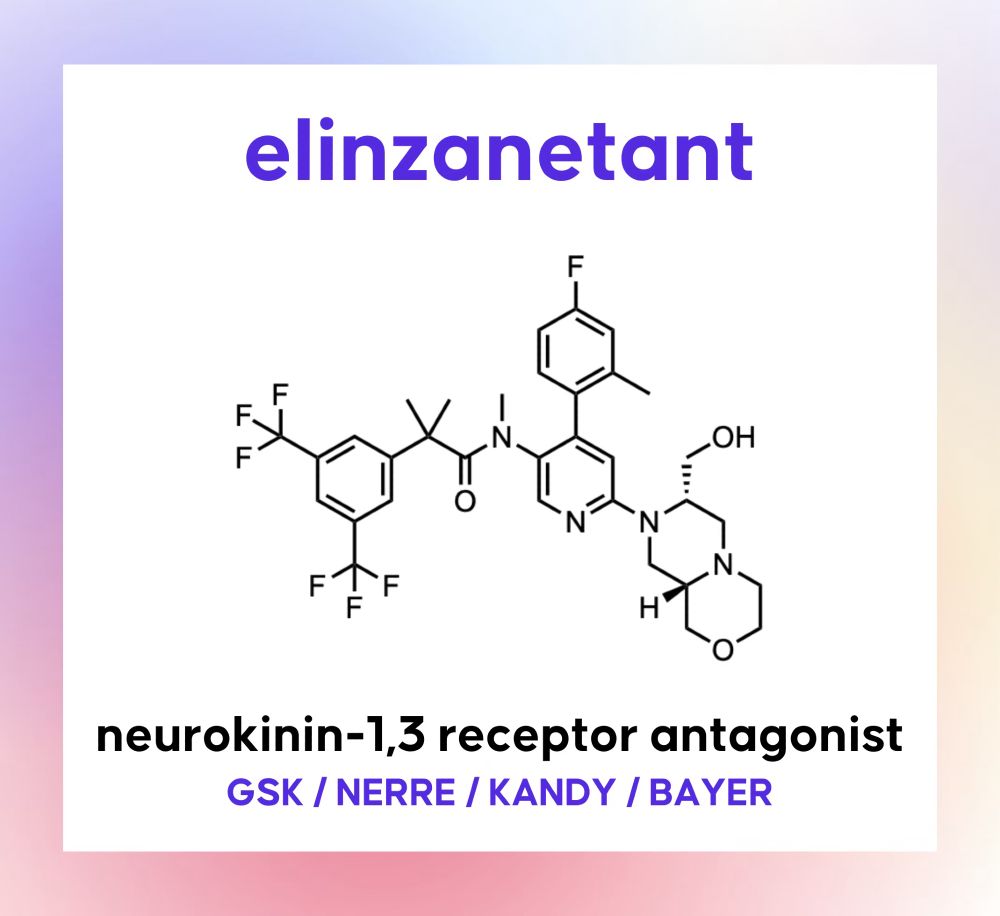
Check out the full case study on Drug Hunter: drughunter.com/molecule/eli...
Matéria completa - regisandrade.com.br/fda-aprova-e...
#Elinzanetant #menopausa #FDA

Matéria completa - regisandrade.com.br/fda-aprova-e...
#Elinzanetant #menopausa #FDA
#NAPCRG #STFM #AAFP #PrimaryCare #FamilyMedicine #EssentialEvidencePlus #InfoPOEMs
spotifycreators-web.app.link/e/50uojPZMdYb
#NAPCRG #STFM #AAFP #PrimaryCare #FamilyMedicine #EssentialEvidencePlus #InfoPOEMs
spotifycreators-web.app.link/e/50uojPZMdYb
💊Those taking the Lynkuet for 12 weeks reported a 73% reduction in the frequency of 𝗵𝗼𝘁 𝗳𝗹𝗮𝘀𝗵𝗲𝘀 & 𝗻𝗶𝗴𝗵𝘁 𝘀𝘄𝗲𝗮𝘁𝘀, compared to a 47% reduction in the placebo group.
💊Those taking the Lynkuet for 12 weeks reported a 73% reduction in the frequency of 𝗵𝗼𝘁 𝗳𝗹𝗮𝘀𝗵𝗲𝘀 & 𝗻𝗶𝗴𝗵𝘁 𝘀𝘄𝗲𝗮𝘁𝘀, compared to a 47% reduction in the placebo group.
#snowdenlitos #Lynkuet #Elinzanetant #MenopauseRelief #NonHormonal #HotFlashRelief #WomensHealth #MenopauseCare #FDAApproved #menopauseeducation #premenopausematters #hormonehealth

#snowdenlitos #Lynkuet #Elinzanetant #MenopauseRelief #NonHormonal #HotFlashRelief #WomensHealth #MenopauseCare #FDAApproved #menopauseeducation #premenopausematters #hormonehealth
Did you know that hot flashes are triggered by tiny chemical messengers in the brain called 𝗻𝗲𝘂𝗿𝗼𝗸𝗶𝗻𝗶𝗻𝘀?
#snowdenlitos #Lynkuet #Elinzanetant #MenopauseRelief #NonHormonal #HotFlashRelief #WomensHealth #MenopauseCare #FDAApproved #womenshealtheducation

Did you know that hot flashes are triggered by tiny chemical messengers in the brain called 𝗻𝗲𝘂𝗿𝗼𝗸𝗶𝗻𝗶𝗻𝘀?
#snowdenlitos #Lynkuet #Elinzanetant #MenopauseRelief #NonHormonal #HotFlashRelief #WomensHealth #MenopauseCare #FDAApproved #womenshealtheducation
🔍 Explore the clinical evidence for Lynkuet at DRUGDOCS® drugdocs.com/drug/Lynkuet
#FDA #PharmaUpdate #MenopauseTreatment #DrugDocs

🔍 Explore the clinical evidence for Lynkuet at DRUGDOCS® drugdocs.com/drug/Lynkuet
#FDA #PharmaUpdate #MenopauseTreatment #DrugDocs

https://www.newsbeep.com/us-pa/26640/
New treatment for menopause hot flashes and night sweats Women who experience menopause symptoms can have them briefly…

https://www.newsbeep.com/us-pa/26640/
New treatment for menopause hot flashes and night sweats Women who experience menopause symptoms can have them briefly…
#FDAapproved Lynkuet (Elinzanetant), a once-daily #nonhormonaltreatment for #moderatetoseverehotflashes&nightsweats arrives Nov 2025.
Hormone-free comfort, balance & confidence.🌿
🔗 snowdenlitos.com/lynkuet-blog/
#Lynkuet #MenopauseRelief #NonHormonal #WomensHealth

#FDAapproved Lynkuet (Elinzanetant), a once-daily #nonhormonaltreatment for #moderatetoseverehotflashes&nightsweats arrives Nov 2025.
Hormone-free comfort, balance & confidence.🌿
🔗 snowdenlitos.com/lynkuet-blog/
#Lynkuet #MenopauseRelief #NonHormonal #WomensHealth

Seattle, WA – October 24, 2025 – Seattle Clinical Research Center (SCRC) is proud to recognize the recent FDA approval of elinzanetant (brand…
Seattle, WA – October 24, 2025 – Seattle Clinical Research Center (SCRC) is proud to recognize the recent FDA approval of elinzanetant (brand…
Seattle, WA – October 24, 2025 – Seattle Clinical Research Center (SCRC) is proud to recognize the recent FDA approval of elinzanetant (brand…
Seattle, WA – October 24, 2025 – Seattle Clinical Research Center (SCRC) is proud to recognize the recent FDA approval of elinzanetant (brand…

