
Antibiotic mechanisms| Cryo-EM | Bacterial pathogens | Membrane proteins
doi.org/10.1101/2025...
This is work from my PhD in the lab of @rhyswg.bsky.social and many collaborators who contributed amazing data!

doi.org/10.1101/2025...
This is work from my PhD in the lab of @rhyswg.bsky.social and many collaborators who contributed amazing data!


Our work, led by @fabianmunder.bsky.social, shows that bacteria from 22 phyla use the high-affinity transporter PqqU to obtain the redox cofactor PQQ from the environment as an alternative to cofactor synthesis.
www.science.org/doi/10.1126/...
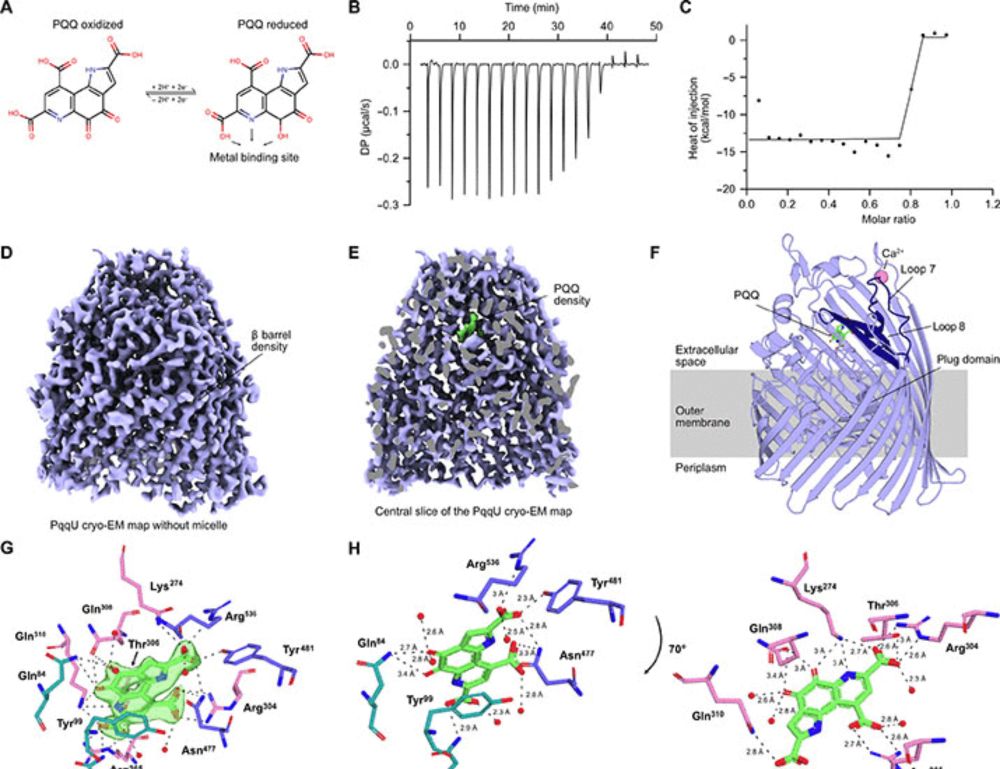
Our work, led by @fabianmunder.bsky.social, shows that bacteria from 22 phyla use the high-affinity transporter PqqU to obtain the redox cofactor PQQ from the environment as an alternative to cofactor synthesis.
www.science.org/doi/10.1126/...

