www.science.org/doi/10.1126/...

www.science.org/doi/10.1126/...
Thanks to everyone involved!
www.cell.com/cell-metabol...
Thanks to everyone involved!
www.cell.com/cell-metabol...
👉https://rdcu.be/d8atv
www.nature.com/articles/s41...
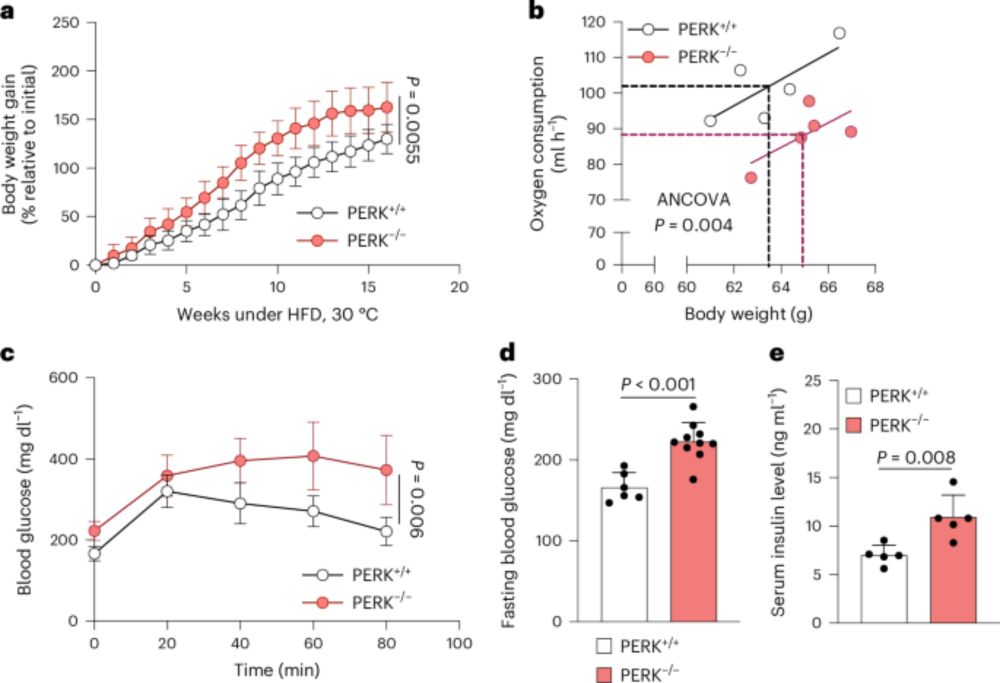
👉https://rdcu.be/d8atv
www.nature.com/articles/s41...
Puigserver and team show that genetic targeting of specific mitochondrial respiratory complex I subunits in melanoma and breast cancer cells boosts tumor immune surveillance via upregulation of antigen-processing and presentation components.
👇
www.nature.com/articles/s43...

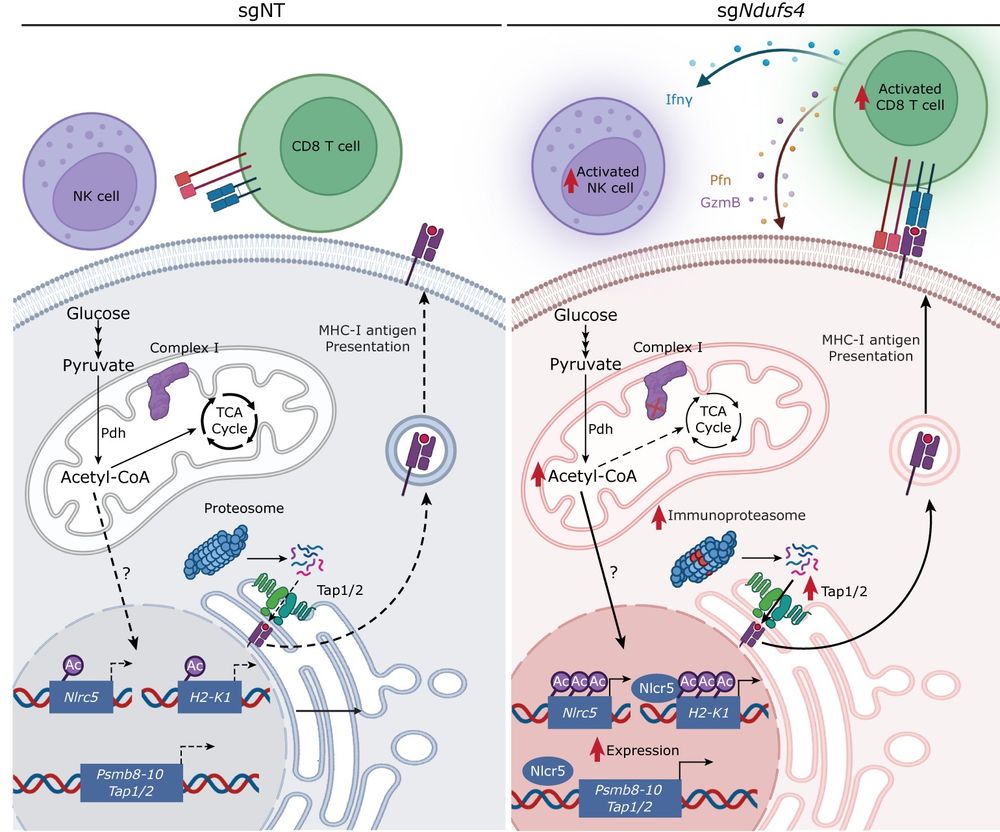
www.nature.com/articles/s43...
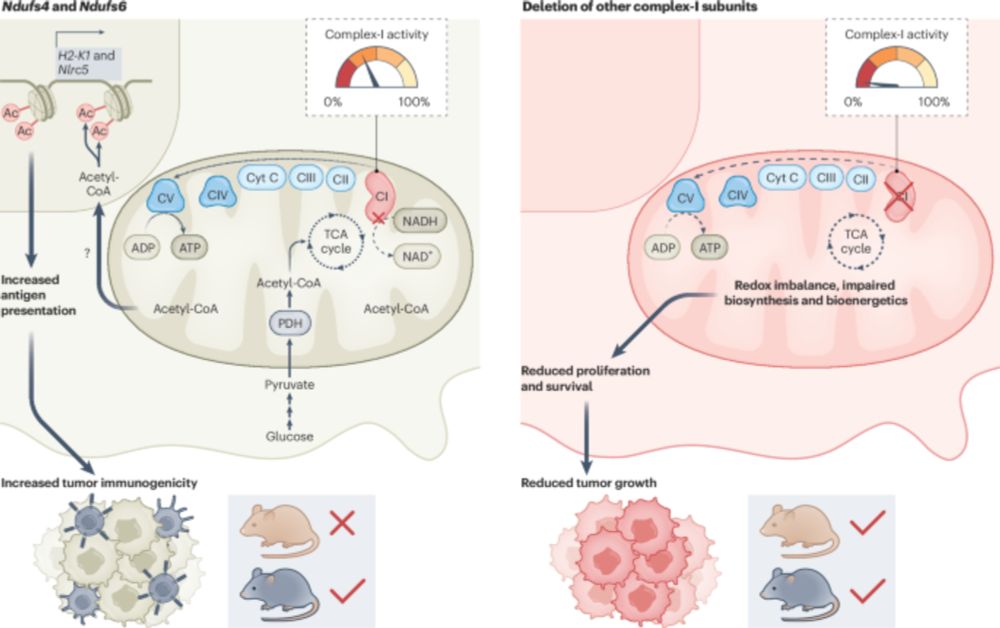
www.nature.com/articles/s43...
rdcu.be/d5ptF
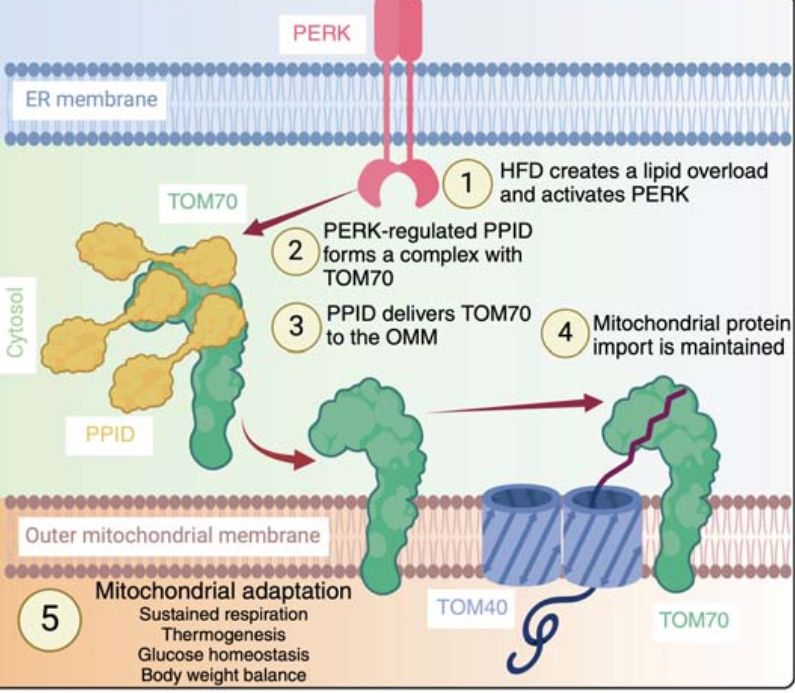
blog.dana-farber.org/insight/2024...
blog.dana-farber.org/insight/2024...


