The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors | PNAS #glycotime www.pnas.org/doi/10.1073/...

pubs.acs.org/doi/10.1021/...
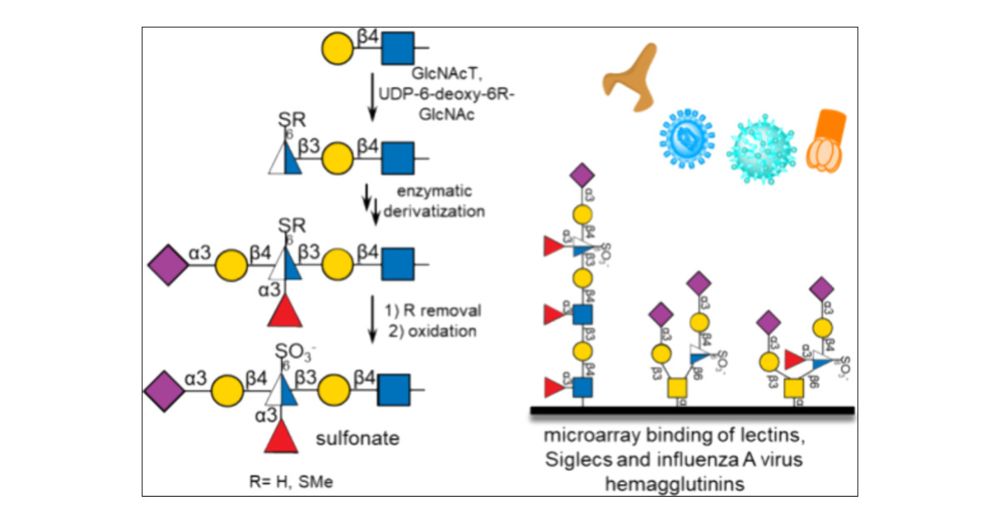
pubs.acs.org/doi/10.1021/...
We find pleiotropic effects of mutations on these phenotypes shape evolution: epistasis alleviates cell-entry but not stability constraints
www.biorxiv.org/content/10.1...



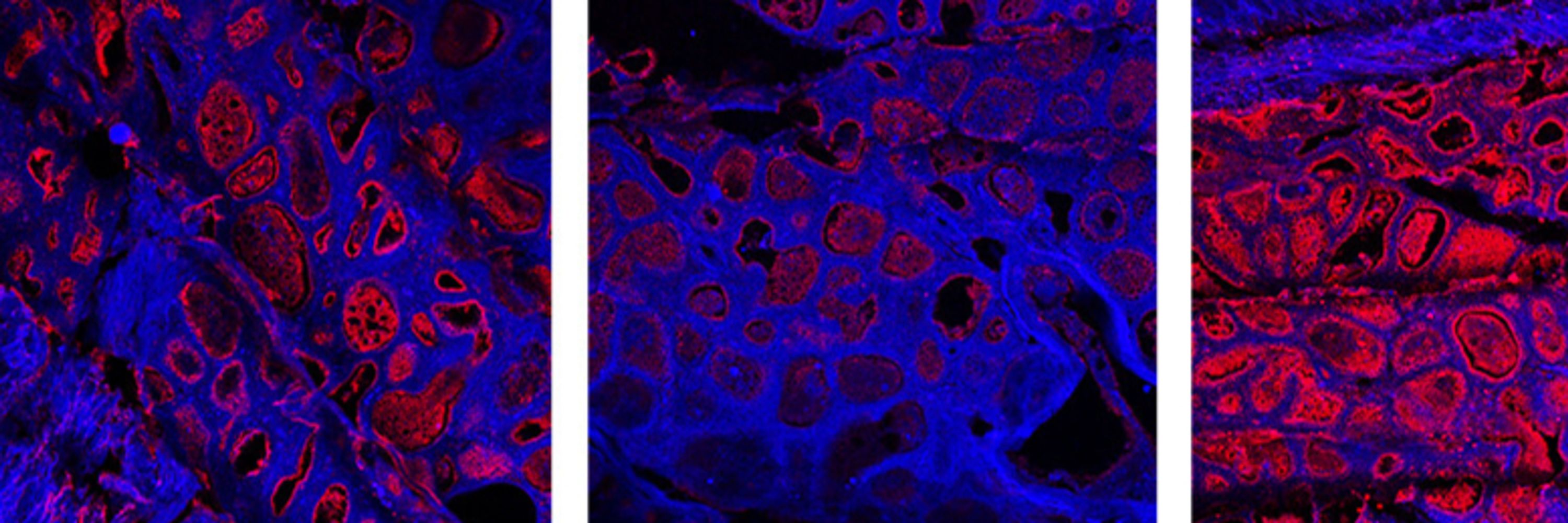
Characterization of the glycoproteins of novel fish influenza B-like viruses #glycotime #lovevirology @uu-cbdd.bsky.social www.biorxiv.org/content/10.1...

Characterization of the glycoproteins of novel fish influenza B-like viruses #glycotime #lovevirology @uu-cbdd.bsky.social www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

www.biorxiv.org/content/10.1...
www.pnas.org/doi/10.1073/...
www.pnas.org/doi/10.1073/...
Interested in virology, stem-cell derived models, glycans?
Apply before 29th of October!
@thijskuiken.bsky.social @lisabauervirus.bsky.social @marionkoopmans.bsky.social @rpdevrieslab.bsky.social
www.werkenbijerasmusmc.nl/en/vacancy/1...

Interested in virology, stem-cell derived models, glycans?
Apply before 29th of October!
@thijskuiken.bsky.social @lisabauervirus.bsky.social @marionkoopmans.bsky.social @rpdevrieslab.bsky.social
www.werkenbijerasmusmc.nl/en/vacancy/1...
www.nature.com/articles/s41...
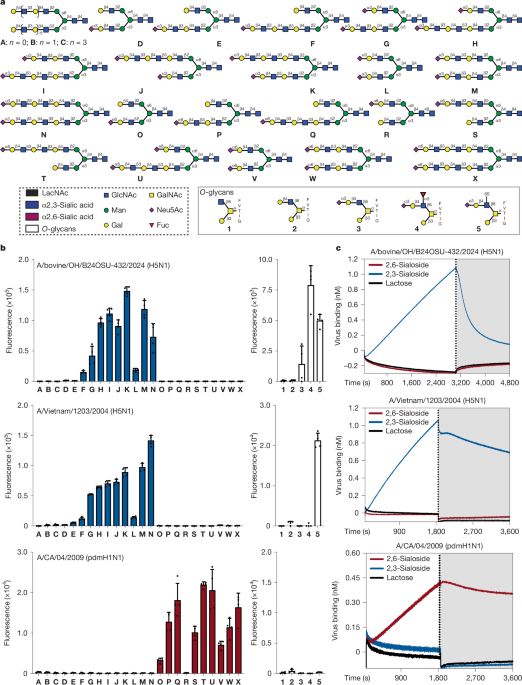
www.nature.com/articles/s41...
The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors | PNAS #glycotime www.pnas.org/doi/10.1073/...
The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors | PNAS #glycotime www.pnas.org/doi/10.1073/...
Follow our progress here or via our Linked-In account: NWO-XL Consortium Viruses like it Sweet
Follow our progress here or via our Linked-In account: NWO-XL Consortium Viruses like it Sweet
🍬Glycans
🦠Viruses
🫁🧠Organoids
🧬 Genetic Engineering
Apply here 👇
www.werkenbijerasmusmc.nl/en/vacancy/1...



>300 milk oligosaccharides in grey seals, analyzed over the lactation period (+metabolomics! +functional experiments!)
Next to increasing known milk glycans by >20% we also present mega-milk glycans (28 sugars!!)
www.biorxiv.org/content/10.1...

>300 milk oligosaccharides in grey seals, analyzed over the lactation period (+metabolomics! +functional experiments!)
Next to increasing known milk glycans by >20% we also present mega-milk glycans (28 sugars!!)
www.biorxiv.org/content/10.1...

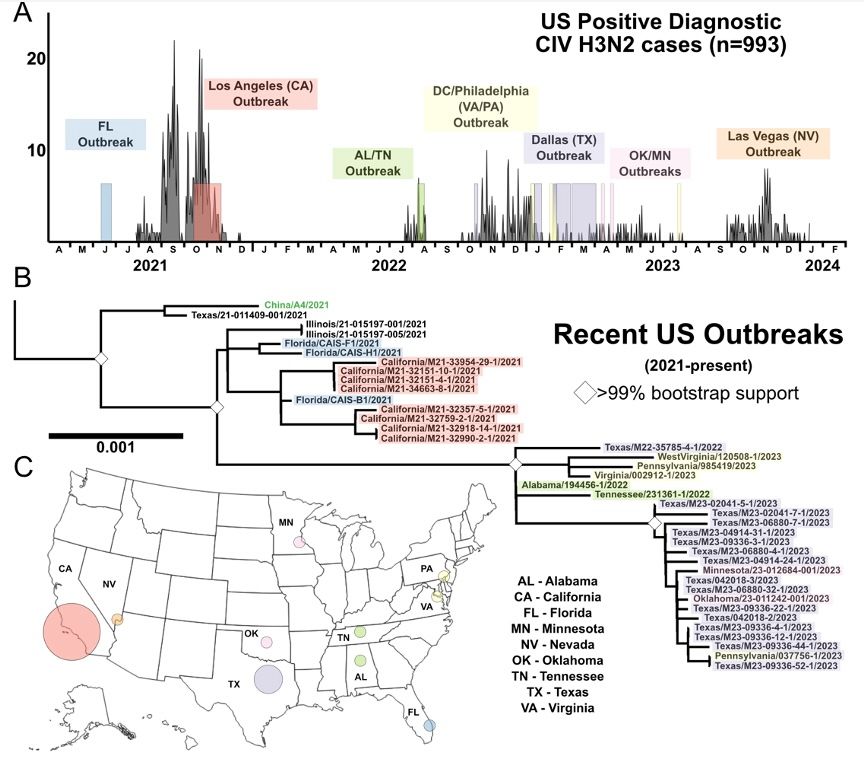
#Avian #influenza remains a serious threat. Strengthening #OneHealth surveillance and response is critical. #BirdFlu


#Avian #influenza remains a serious threat. Strengthening #OneHealth surveillance and response is critical. #BirdFlu




