@tykwinskigroup.bsky.social
370 followers
130 following
14 posts
carbon (mostly of the sp variety), physical organic chemistry, cool molecules, and photophysics of conjugated molecules
Posts
Media
Videos
Starter Packs
Reposted
Reposted
Bettinger Lab
@bettingerlab.bsky.social
· Jul 28

High energy density dihydroazaborinine dyads and triad for molecular solar thermal energy storage
The reversible photoisomerization of 1,2-dihydro-1,2-azaborinines (BN benzenes) to their Dewar isomers (2-aza-3-borabicyclo[2.2.0]hex-5-enes) provides a promising platform for molecular solar thermal ...
doi.org
Reposted
Luis Campos
@soyluiscampos.bsky.social
· Jul 17
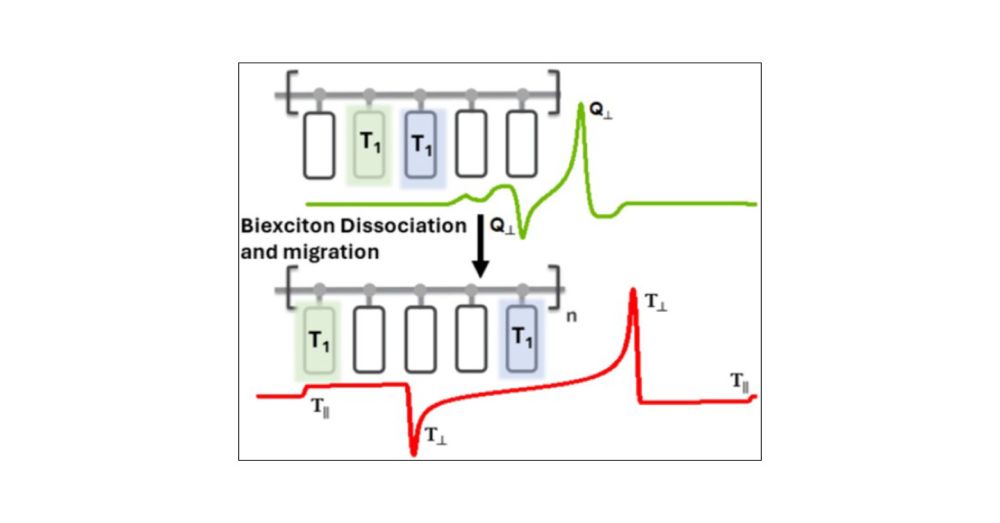
Elucidating Quintet-State Dynamics in Singlet Fission Oligomers and Polymers with Tetracene Pendants
To unlock the potential of molecular engineering for practical quantum sensing and computing, it is essential to create and control pure magnetic states in molecular systems. Singlet fission (SF) in o...
pubs.acs.org
Reposted
Reposted
Guldi Group
@guldi-group.bsky.social
· Apr 17

Reversible gating of singlet fission by tuning the role of a charge-transfer state - Nature Communications
Singlet fission is an efficient way to generate excitons but is rarely responsive to external stimuli. Here the authors design a linked tetracene dimer where acid/base interactions can control singlet...
doi.org
Reposted
Reposted
Reposted
Art Winter
@arthurhwinter.bsky.social
· Mar 7
Automerization of an Enediyne via a Symmetrical p-Benzyne Diradical Intermediate
Automerization is defined as a rearrangement reaction that yields a degenerate form of the starting material. We now report that cyclohexeno[3,4]cyclodeca-1,5-diyne-3-ene rearranges to its automer, via a D2h-symmetric p-benzyne intermediate. The NMR evidence is that when the enediyne is heated to 75 °C in DMSO with either LiI or NaNO3 as possible nucleophile, the enediyne is consumed only in the presence of LiI, whereas it remains “unchanged” in the presence of NaNO3.
pubs.acs.org
Reposted
Reposted