Adam Gilbert
@adammgilbert65.bsky.social
230 followers
200 following
87 posts
Executive Director of Medicinal Chemistry at Pfizer. Novel drug discovery strategies #proteindegradation #covalents #peptides #dels #vaccines #PFEcolleague
Posts
Media
Videos
Starter Packs
Reposted by Adam Gilbert
Adam Gilbert
@adammgilbert65.bsky.social
· Aug 31

Data-driven Design of PROTAC Linkers to Improve PROTAC Cell Membrane Permeability
Proteolysis-targeting chimeras (PROTACs) are promising next-generation therapeutics for the degradation of disease-associated proteins. However, optimizing the physicochemical properties of PROTACs, p...
doi.org
Adam Gilbert
@adammgilbert65.bsky.social
· Aug 12

Selectivity Profiling of Bromodomain PROTACs Using Chemical Inducers of Proximity DNA-Encoded Library Screening
Chemical Inducers of Proximity DNA-Encoded Library (CIP-DEL) screening enables high-throughput discovery of compounds that induce protein–protein interactions, including Proteolysis-Targeting Chimeras...
doi.org
Reposted by Adam Gilbert
Carl T. Bergstrom
@carlbergstrom.com
· Aug 12

RFK Jr. in interview with Scripps News: ‘Trusting the experts is not science’
HHS Secretary RFK Jr. sat down with Scripps News for a wide-ranging interview, discussing mRNA vaccine funding policy changes and a recent shooting at the Centers for Disease Control and Prevention.
www.scrippsnews.com
Adam Gilbert
@adammgilbert65.bsky.social
· Jul 31

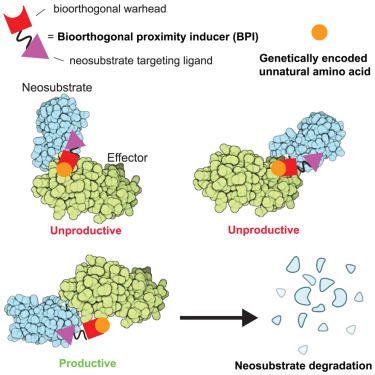
Rewiring DNA repair with PARP-based chemical inducers of proximity
Chemical inducers of proximity (CIPs) can elicit durable, and often neomorphic, biological effects through the formation of a ternary complex, even at low equilibrium occupancy of their targets. This ...
www.biorxiv.org
Adam Gilbert
@adammgilbert65.bsky.social
· Jul 31

The CryoEM Structure of Human GPR75: Insights into ECL2-Mediated Activation
GPR75 is one of the most promising emerging targets for the treatment of obesity and related co-morbidities, as its loss of function directly correlates with a decreased body mass index (BMI) in human...
doi.org
Adam Gilbert
@adammgilbert65.bsky.social
· Jul 23
Adam Gilbert
@adammgilbert65.bsky.social
· Jul 23

Desulfinative Cross-Coupling as a Method to Overcome Problematic Suzuki–Miyaura Reactions of Pharmaceutically Relevant Heteroaromatic Boronates
The well documented difficulties associated with direct (hetero)arylation of aza-aromatics (e.g., azines) at the α-position to nitrogen led to a collaborative project between the Willis group at Oxfor...
pubs.acs.org
Adam Gilbert
@adammgilbert65.bsky.social
· Jun 11
Adam Gilbert
@adammgilbert65.bsky.social
· Jun 11

Covalent Destabilizing Degrader of AR and AR-V7 in Androgen-Independent Prostate Cancer Cells
Androgen-independent prostate cancers, correlated with heightened aggressiveness and poor prognosis, are caused by mutations or deletions in the androgen receptor (AR) or the expression of truncated variants of AR that are constitutively activated. Currently, drugs and drug candidates against AR target the steroid-binding domain to antagonize or degrade AR. However, these compounds cannot therapeutically access largely intrinsically disordered truncated splice variants of AR, such as AR-V7, which only possess the N-terminal transactivation domain and DNA-binding domain and are missing the ligand-binding domain. Targeting intrinsically disordered regions within transcription factors has remained challenging and is considered “undruggable”. Herein, we leverage a cysteine-reactive covalent ligand library in a cellular screen to identify the degraders of AR and AR-V7 in androgen-independent prostate cancer cells. We identified a covalent compound, EN1441, that selectively degrades AR and AR-V7 in a proteasome-dependent manner through direct covalent targeting of intrinsically disordered cysteine C125 in the N-terminal transactivation domain of AR and AR-V7. EN1441 causes significant and selective destabilization of AR and AR-V7, leading to the aggregation of AR/AR-V7 and subsequent proteasome-mediated degradation. Consistent with targeting both AR and AR-V7, we find that EN1441 completely inhibits total AR transcriptional activity in androgen-independent prostate cancer cells expressing both AR and AR-V7 compared with AR antagonists or degraders that only target the ligand-binding domain of full-length AR, such as enzalutamide and ARV-110. Our results put forth a pathfinder molecule EN1441 that targets an intrinsically disordered cysteine within AR to destabilize, degrade, and inhibit both AR and AR-V7 in androgen-independent prostate cancer cells and highlights the utility of covalent ligand discovery approaches in directly targeting, destabilizing, inhibiting, and degrading classically undruggable transcription factor targets.
doi.org
Reposted by Adam Gilbert