Ashley Sullivan
@aesully98.bsky.social
68 followers
76 following
19 posts
🦬 // PhD candidate in the Aaron Whiteley Lab at CU Boulder. she/her
Posts
Media
Videos
Starter Packs
Reposted by Ashley Sullivan
Reposted by Ashley Sullivan
Reposted by Ashley Sullivan
Reposted by Ashley Sullivan
Reposted by Ashley Sullivan
Amy Conte
@amyconte.bsky.social
· Jun 4
PLOS Biology
@plosbiology.org
· Jun 4
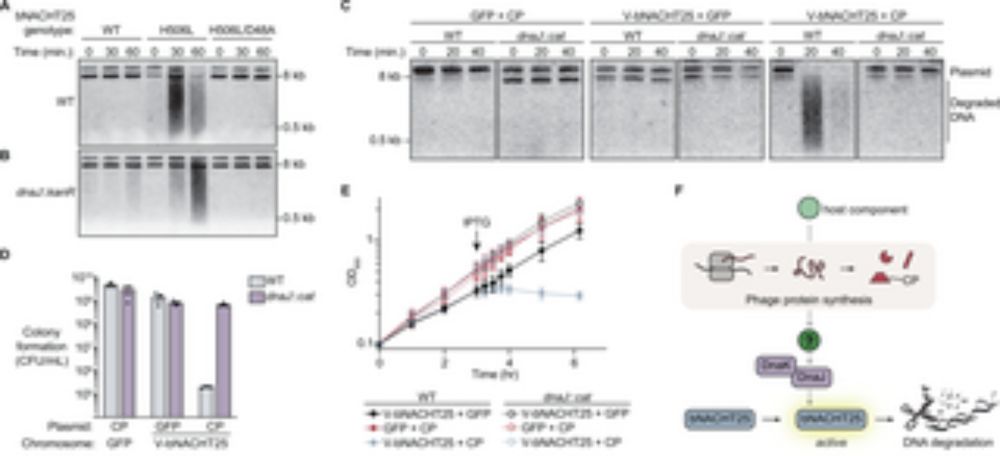
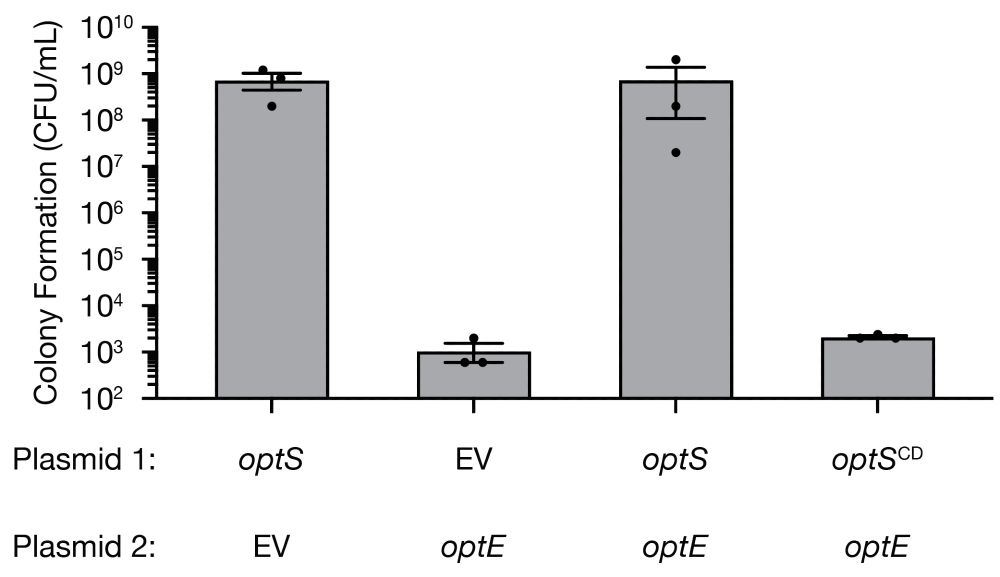
DnaJ mediates phage sensing by the bacterial NLR-related protein bNACHT25
Bacteria encode homologs of the human NACHT proteins that form inflammasomes, and use them to defend against phage infection. This study shows that the NLR-related protein bNACHT25 detects diverse pha...
plos.io
Reposted by Ashley Sullivan
Ben Morehouse
@benmorehouse.bsky.social
· Mar 31
Ashley Sullivan
@aesully98.bsky.social
· Mar 31

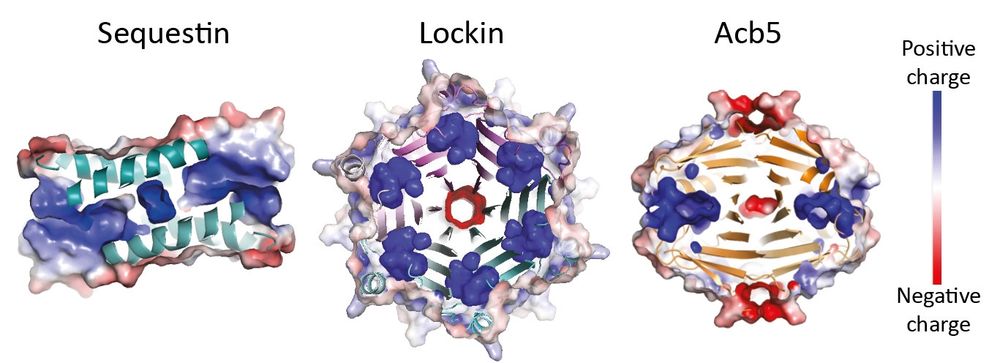
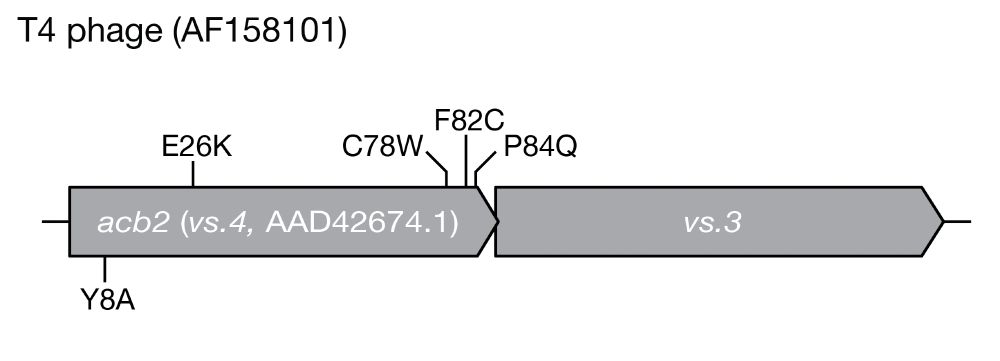
A minimal CRISPR polymerase produces decoy cyclic nucleotides to detect phage anti-defense proteins
Bacteria use antiphage systems to combat phages, their ubiquitous competitors, and evolve new defenses through repeated reshuffling of basic functional units into novel reformulations. A common theme ...
www.biorxiv.org
Ashley Sullivan
@aesully98.bsky.social
· Mar 31

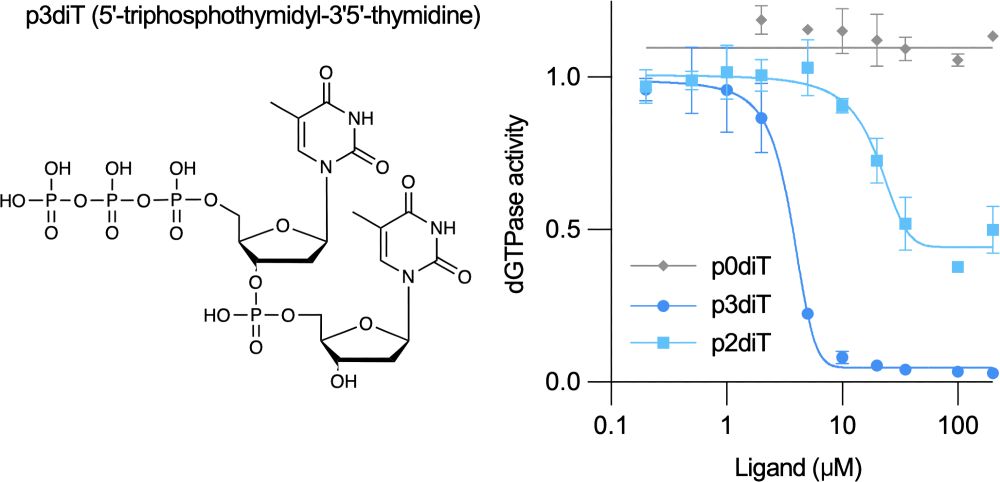
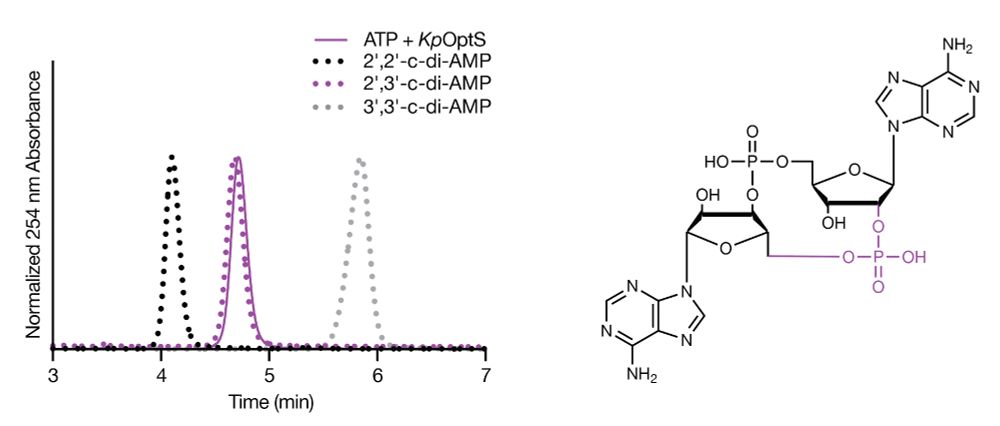
A miniature CRISPR-Cas10 enzyme confers immunity by an inverse signaling pathway
Microbial and viral co-evolution has created immunity mechanisms involving oligonucleotide signaling that share mechanistic features with human anti-viral systems. In these pathways, including CBASS a...
www.biorxiv.org
Ashley Sullivan
@aesully98.bsky.social
· Mar 31
Ashley Sullivan
@aesully98.bsky.social
· Mar 31
Ashley Sullivan
@aesully98.bsky.social
· Mar 31
Ashley Sullivan
@aesully98.bsky.social
· Mar 31
Ashley Sullivan
@aesully98.bsky.social
· Mar 31
Ashley Sullivan
@aesully98.bsky.social
· Mar 31