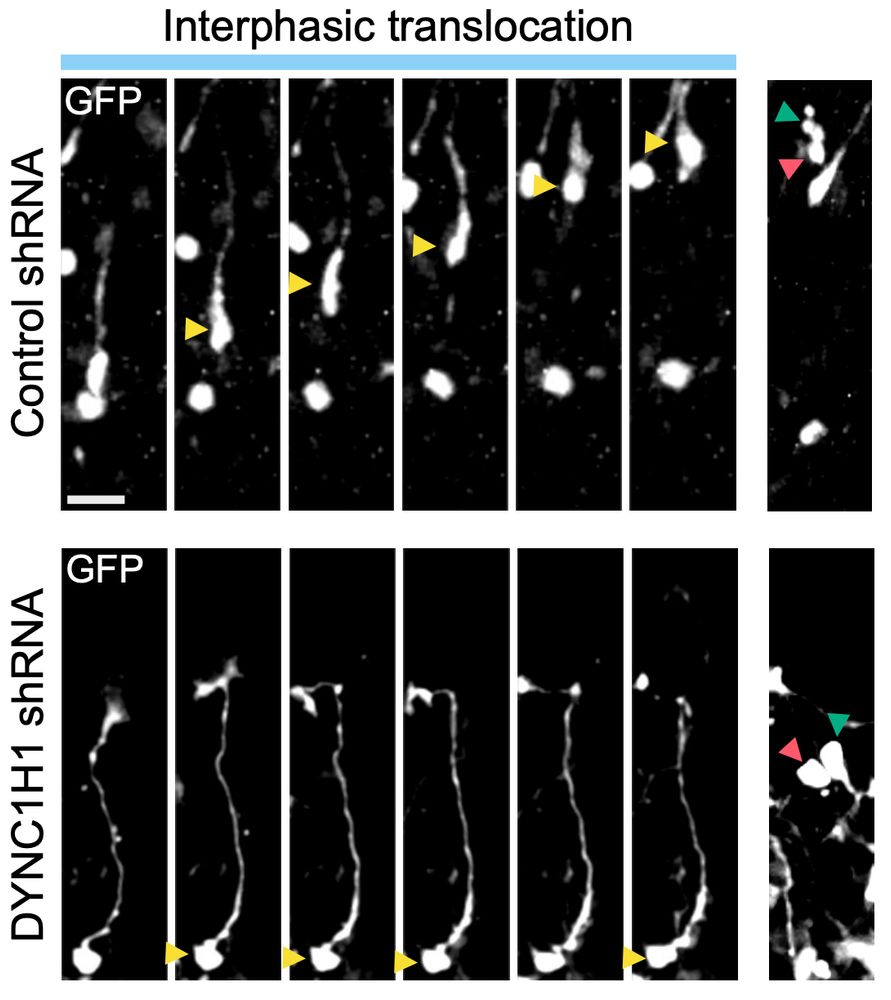
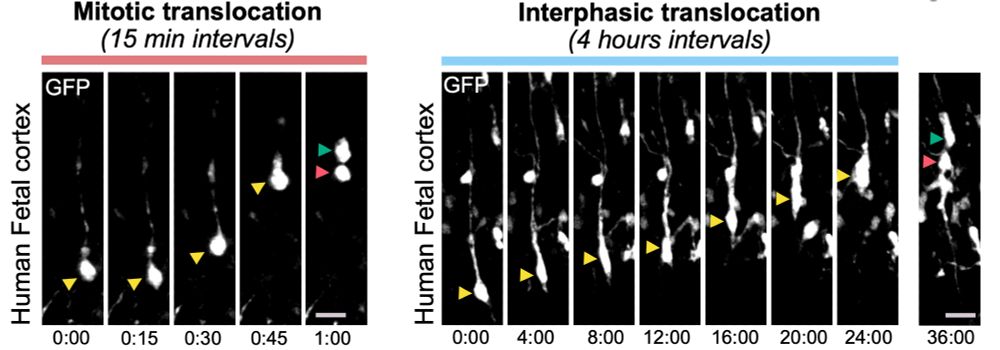
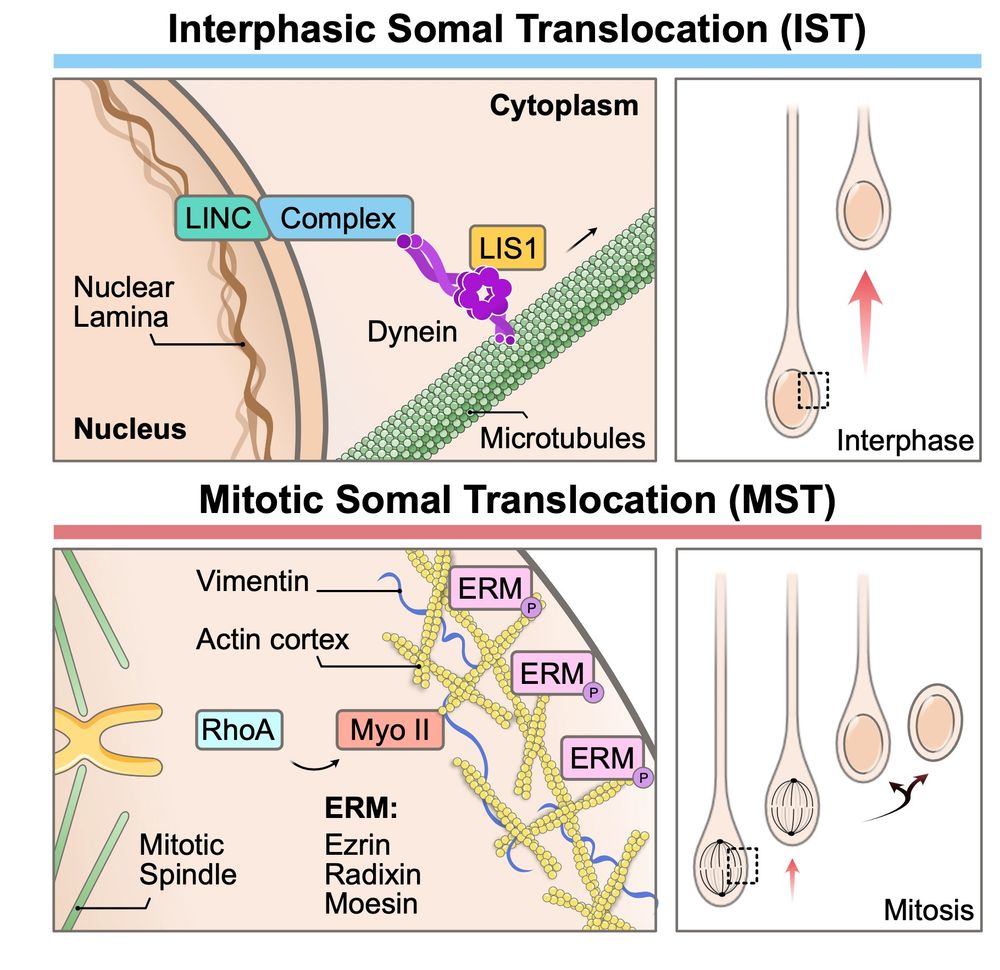
Alexandre Baffet
@alexbaffet.bsky.social
250 followers
310 following
25 posts
Cell Biologist studying neurogenesis and neural stem cells using live imaging methods.
INSERM investigator. Group leader @institut_curie Paris.
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Alexandre Baffet
Brendan Evano
@brendan-evano.bsky.social
· Mar 14

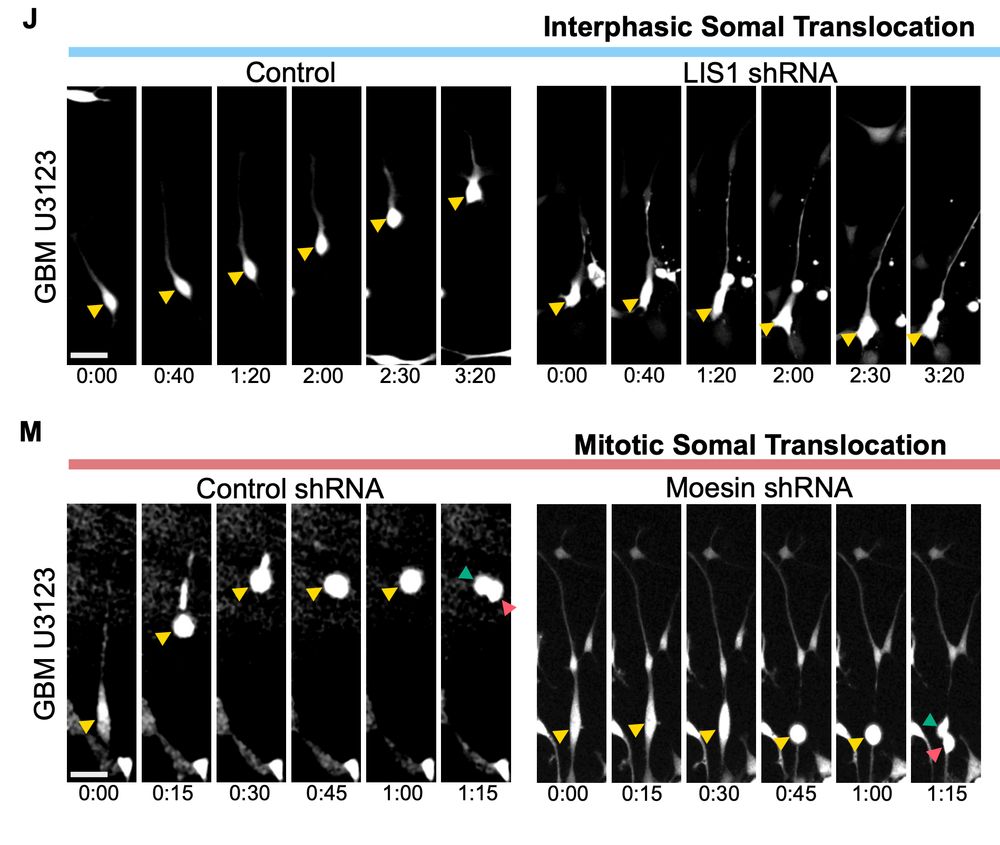
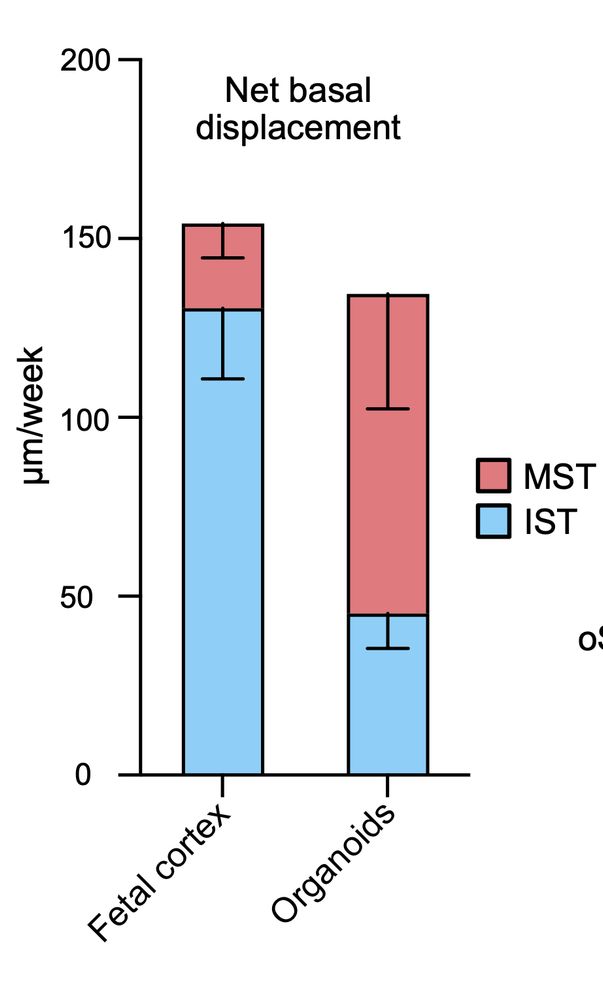
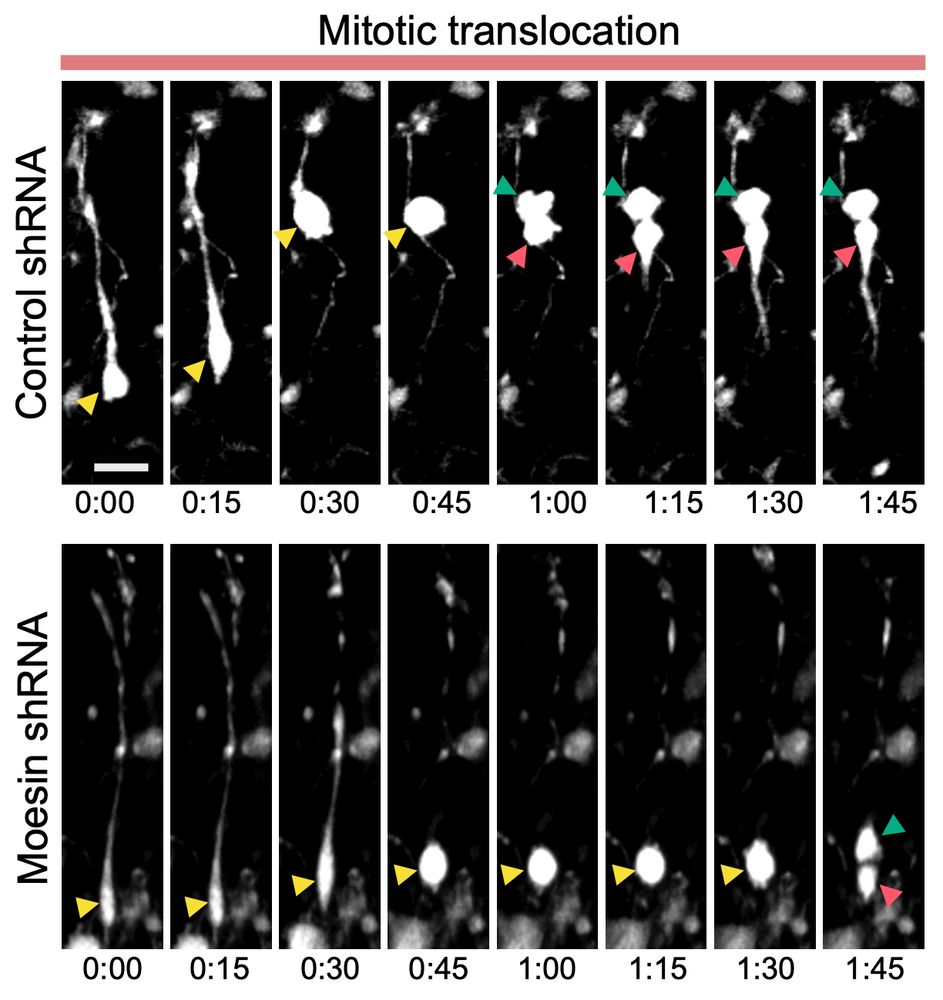
Impaired stem cell migration and divisions in Duchenne Muscular Dystrophy revealed by live imaging
Dysregulation of stem cell properties is a hallmark of many pathologies, but the dynamic behaviour of stem cells in their microenvironment during disease progression remains poorly understood. Using t...
www.biorxiv.org
Alexandre Baffet
@alexbaffet.bsky.social
· Mar 14
Reposted by Alexandre Baffet
Alexandre Baffet
@alexbaffet.bsky.social
· Jan 13
Alexandre Baffet
@alexbaffet.bsky.social
· Jan 10
Reposted by Alexandre Baffet
Li Wang
@liwangneuro.bsky.social
· Jan 9
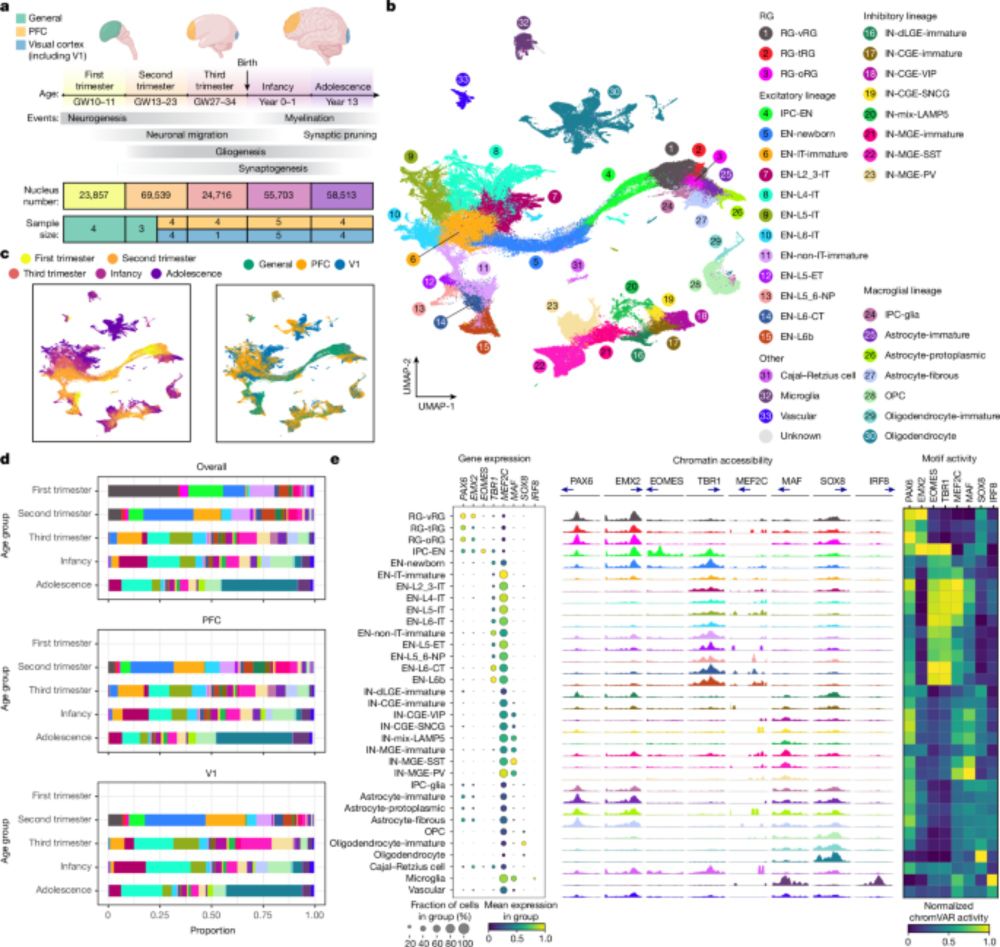
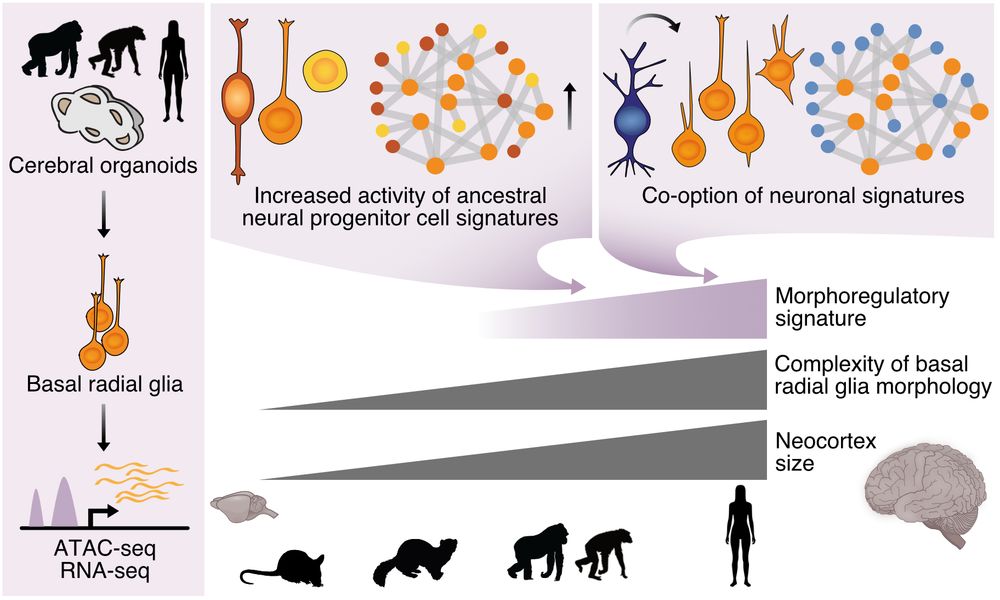
Molecular and cellular dynamics of the developing human neocortex - Nature
Tripotential intermediate progenitor cells are responsible for the local production of GABAergic neurons, oligodendrocyte precursor cells and astrocytes in the human neocortex.
www.nature.com
Alexandre Baffet
@alexbaffet.bsky.social
· Jan 10
Alexandre Baffet
@alexbaffet.bsky.social
· Jan 10