Alex Kalinin
@alxndrkalinin.bsky.social
250 followers
250 following
25 posts
AI/ML for image-based profiling @broadinstitute.org | prev CUHK-SZ & UMich
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Alex Kalinin
Reposted by Alex Kalinin
Alex Kalinin
@alxndrkalinin.bsky.social
· Jul 12
Alex Kalinin
@alxndrkalinin.bsky.social
· Jul 10

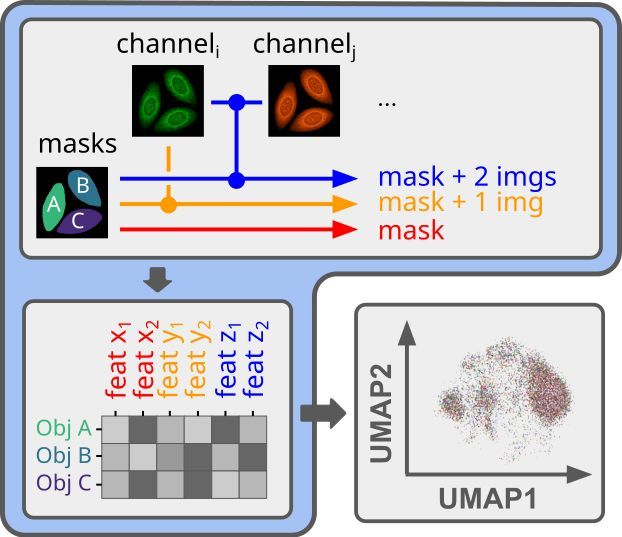
Foreground-aware Virtual Staining for Accurate 3D Cell Morphological Profiling
Microscopy enables direct observation of cellular morphology in 3D, with transmitted-light methods offering low-cost, minimally invasive imaging and fluorescence microscopy providing specificity and c...
arxiv.org
Alex Kalinin
@alxndrkalinin.bsky.social
· Jul 10
Alex Kalinin
@alxndrkalinin.bsky.social
· Jul 10
Reposted by Alex Kalinin
Reposted by Alex Kalinin
Alex Kalinin
@alxndrkalinin.bsky.social
· Jun 23
Reposted by Alex Kalinin
Alex Kalinin
@alxndrkalinin.bsky.social
· Jun 10
Alex Kalinin
@alxndrkalinin.bsky.social
· Jun 10
Alex Kalinin
@alxndrkalinin.bsky.social
· Jun 10