Amy Weeks
@amyweeks.bsky.social
1.6K followers
310 following
22 posts
Asst Prof @ UW-Madison Biochemistry. Protein Engineering, Chemical Biology, Proteomics, Proteases, Enzymology.
Posts
Media
Videos
Starter Packs
Reposted by Amy Weeks
Reposted by Amy Weeks
Jeff Martell
@jeffmartell.bsky.social
· Aug 14
DNA-Scaffolded Ultrahigh-Throughput Reaction Screening
Discovering and optimizing reactions is central to synthetic chemistry. However, chemical reactions are traditionally screened using relatively low-throughput methods, prohibiting exploration of diver...
chemrxiv.org
Amy Weeks
@amyweeks.bsky.social
· Aug 13

An atlas of substrate specificities for the human serine/threonine kinome - Nature
Analysis of the kinase activity of 300 protein Ser/Thr kinases reveals that the substrate specificity of the kinome is substantially more diverse than expected and is driven extensively by negative se...
www.nature.com
Amy Weeks
@amyweeks.bsky.social
· Aug 13

An atlas of substrate specificities for the human serine/threonine kinome - Nature
Analysis of the kinase activity of 300 protein Ser/Thr kinases reveals that the substrate specificity of the kinome is substantially more diverse than expected and is driven extensively by negative se...
www.nature.com
Reposted by Amy Weeks
coonlab.bsky.social
@coonlab.bsky.social
· Jul 30

SynchroSep-MS: Parallel LC Separations for Multiplexed Proteomics
Achieving high throughput remains a challenge in MS-based proteomics for large-scale applications. We introduce SynchroSep-MS, a novel method for parallelized, label-free proteome analysis that leverages the rapid acquisition speed of modern mass spectrometers. This approach employs multiple liquid chromatography columns, each with an independent sample, simultaneously introduced into a single mass spectrometer inlet. A precisely controlled retention time offset between sample injections creates distinct elution profiles, facilitating unambiguous analyte assignment. We modified the DIA-NN workflow to effectively process these unique parallelized data, accounting for retention time offsets. Using a dual-column setup with mouse brain peptides, SynchroSep-MS detected approximately 16,700 unique protein groups, nearly doubling the peptide information obtained from a conventional single proteome analysis. The method demonstrated excellent precision and reproducibility (median protein %RSDs less than 4%) and high quantitative linearity (median R2 greater than 0.96) with minimal matrix interference. SynchroSep-MS represents a new paradigm for data collection and the first example of label-free multiplexed proteome analysis via parallel LC separations, offering a direct strategy to accelerate throughput for demanding applications such as large-scale clinical cohorts and single-cell analyses without compromising peak capacity or causing ionization suppression.
pubs.acs.org
Amy Weeks
@amyweeks.bsky.social
· Jul 18
Amy Weeks
@amyweeks.bsky.social
· Jul 18
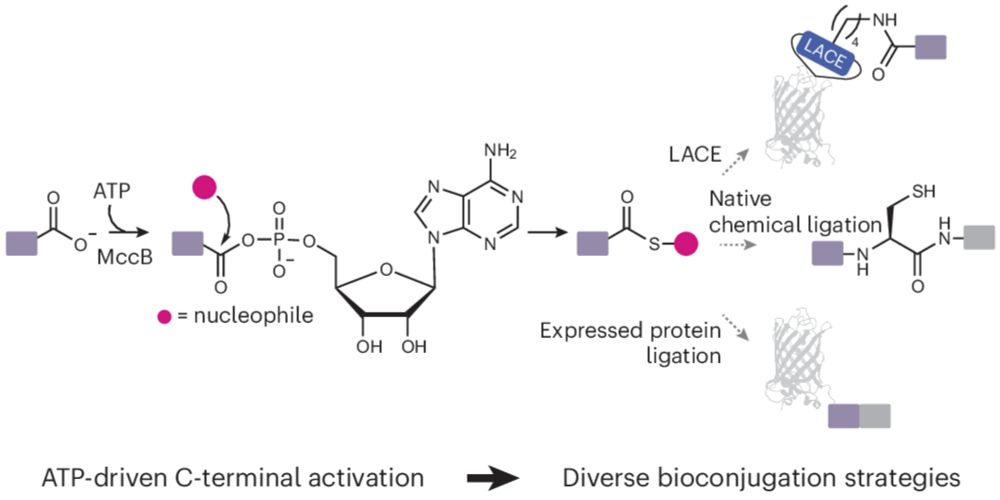
Engineered reactivity of a bacterial E1-like enzyme enables ATP-driven modification of protein and peptide C termini
Nature Chemistry - In living systems, ATP provides an energetic driving force for protein synthesis and modification. Now, an engineered enzymatic tool has been developed for high-yield, ATP-driven...
rdcu.be
Reposted by Amy Weeks
Reposted by Amy Weeks
Neel Shah
@nshahlab.bsky.social
· Jun 21
Reposted by Amy Weeks
Reposted by Amy Weeks
Reposted by Amy Weeks
Reposted by Amy Weeks
Scott Coyle
@cellraiser.bsky.social
· Apr 9

Three UW–Madison students named 2025 Goldwater Scholars
Three University of Wisconsin–Madison students have been named winners of 2025 Goldwater Scholarships, the premier undergraduate scholarship in mathematics, engineering and the natural sciences in the...
news.wisc.edu
Reposted by Amy Weeks
UW–Madison
@uwmadison.bsky.social
· Apr 9

Three UW–Madison students named 2025 Goldwater Scholars
Three University of Wisconsin–Madison students have been named winners of 2025 Goldwater Scholarships, the premier undergraduate scholarship in mathematics, engineering and the natural sciences in the...
news.wisc.edu