Asma Farhat
@asmafarhat.bsky.social
200 followers
860 following
15 posts
PhD student @Sylvia Knapp Lab, Vienna
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Asma Farhat
Reposted by Asma Farhat
Reposted by Asma Farhat
BonelliLab
@bonellilab.bsky.social
· Apr 28
Reposted by Asma Farhat
Asma Farhat
@asmafarhat.bsky.social
· Apr 4
Reposted by Asma Farhat
Reposted by Asma Farhat
Asma Farhat
@asmafarhat.bsky.social
· Mar 30
Asma Farhat
@asmafarhat.bsky.social
· Mar 30
Asma Farhat
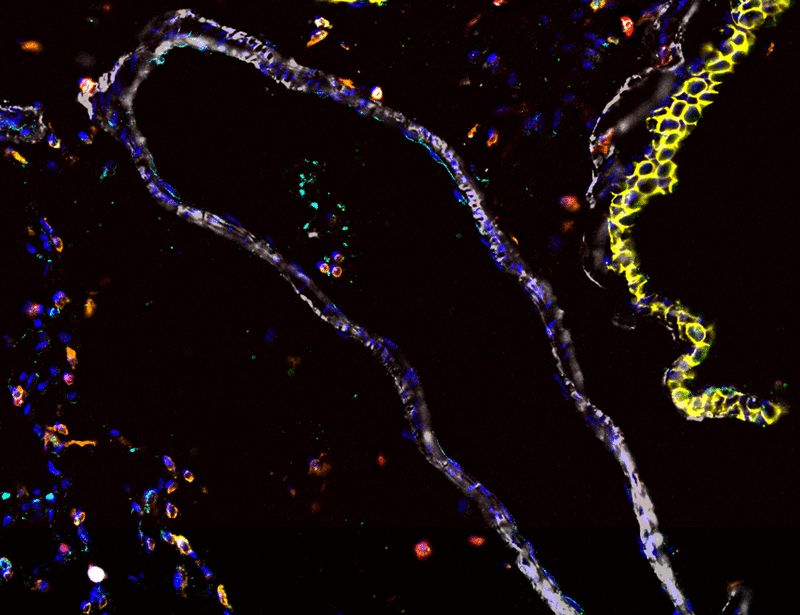
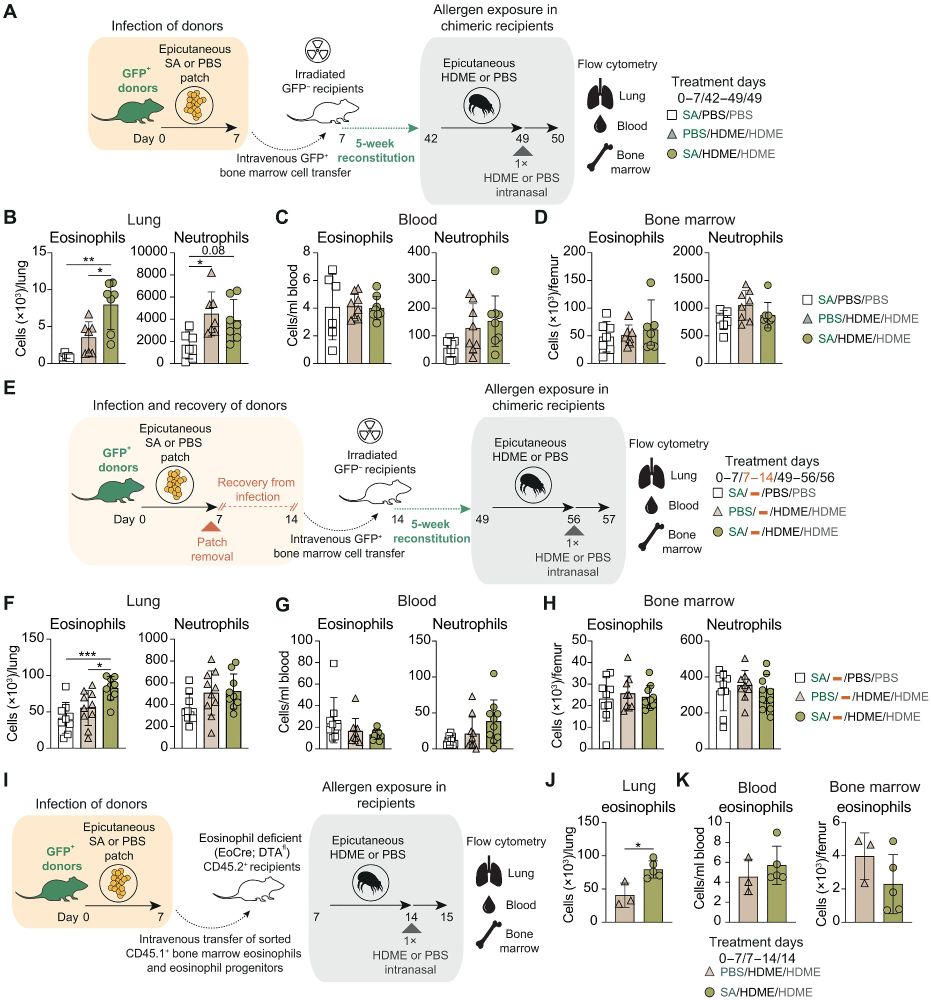
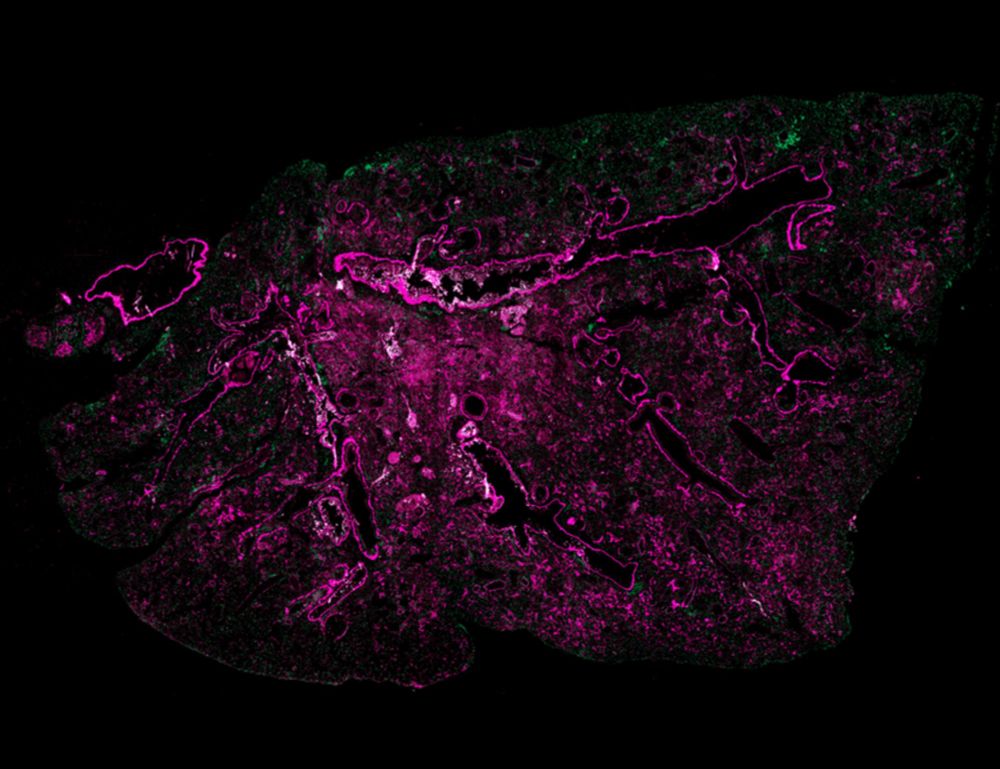
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Asma Farhat
@asmafarhat.bsky.social
· Mar 28
Reposted by Asma Farhat
Mass Lab
@masslab.bsky.social
· Dec 1

Holistic genetic barcoding reveals a lineage tree of tissue macrophage development
Tissue macrophages are crucial for organ development and homeostasis, yet the developmental routes leading to tissue macrophages remain controversial. By combining unbiased Polylox barcoding with comp...
www.biorxiv.org
Reposted by Asma Farhat