Daniel Alber
@danielalber.bsky.social
97 followers
140 following
10 posts
Postdoc, Hughes Lab @ UPenn | PhD, Shvartsman Lab @ Princeton
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Daniel Alber
Reposted by Daniel Alber
Reposted by Daniel Alber
Reposted by Daniel Alber
Reposted by Daniel Alber
Daniel Alber
@danielalber.bsky.social
· Jun 12
Reposted by Daniel Alber
Reposted by Daniel Alber
Eszter Posfai
@eposfai.bsky.social
· Mar 22
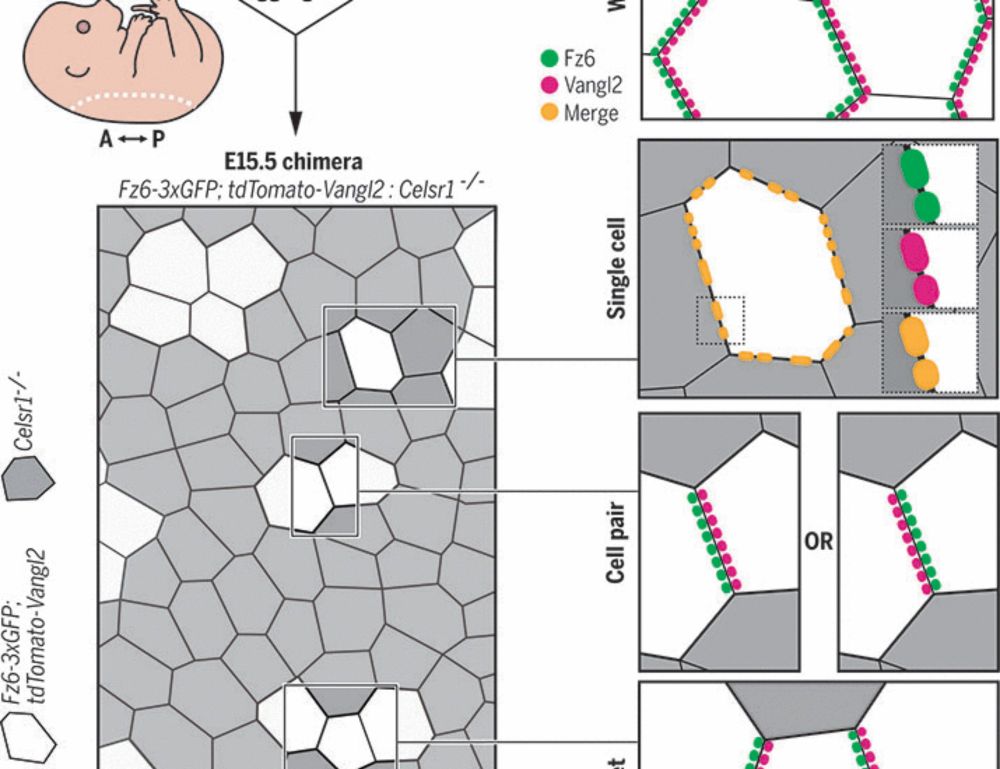
Epithelial polarization by the planar cell polarity complex is exclusively non–cell autonomous
For cells to polarize collectively along a tissue plane, asymmetrically localized planar cell polarity (PCP) complexes must form intercellular contacts between neighboring cells. Yet, it is unknown wh...
www.science.org
Reposted by Daniel Alber
Reposted by Daniel Alber
Arthur Michaut 🔬🐣
@amichaut.bsky.social
· Mar 13
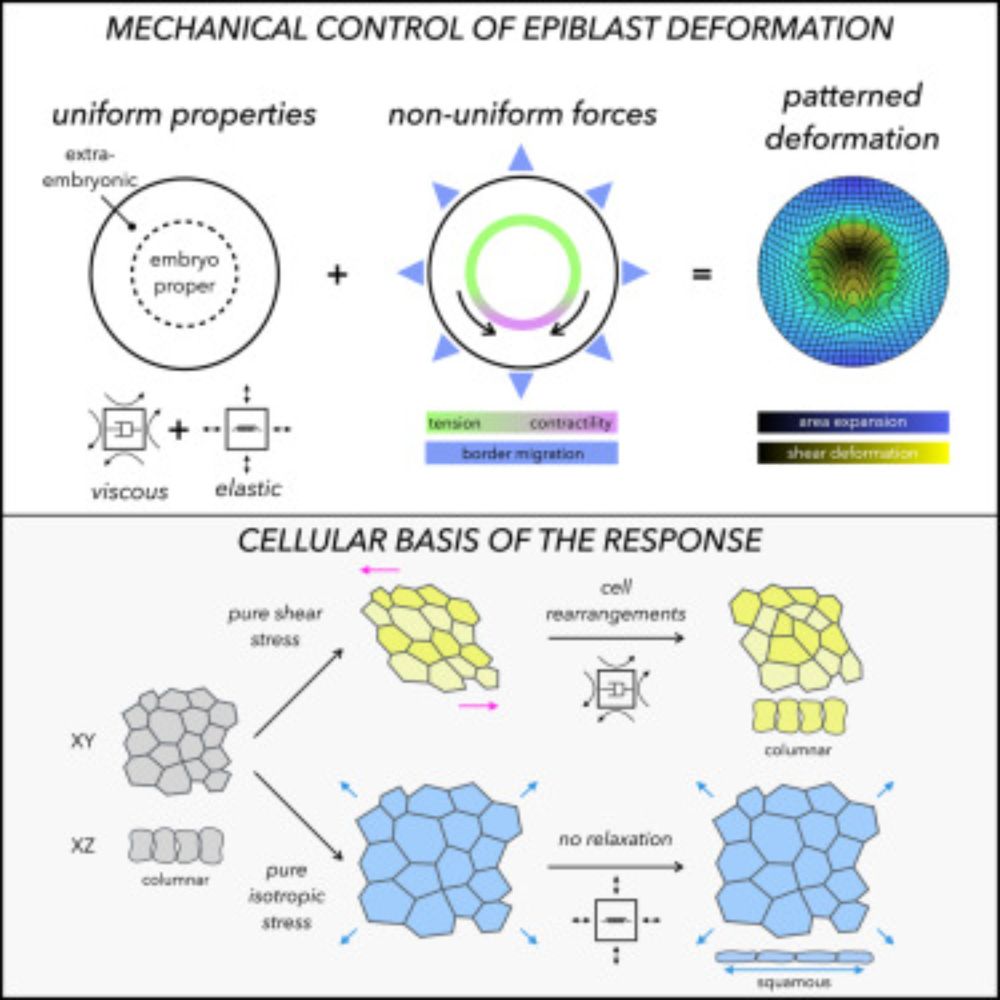
A tension-induced morphological transition shapes the avian extra-embryonic territory
Michaut et al. show that the tension produced during epiboly elastically stretches
the extra-embryonic (EE) territory, while the embryo proper (EP) remains unaffected.
Although the whole epiblast (EP ...
www.cell.com
Reposted by Daniel Alber
Reposted by Daniel Alber
Reposted by Daniel Alber