Eszter Posfai
@eposfai.bsky.social
500 followers
200 following
16 posts
Preimplantation development and germline biology. Live imaging and genome engineering. Assistant Prof at Princeton.
Posts
Media
Videos
Starter Packs
Reposted by Eszter Posfai
Reposted by Eszter Posfai
Eszter Posfai
@eposfai.bsky.social
· Aug 2
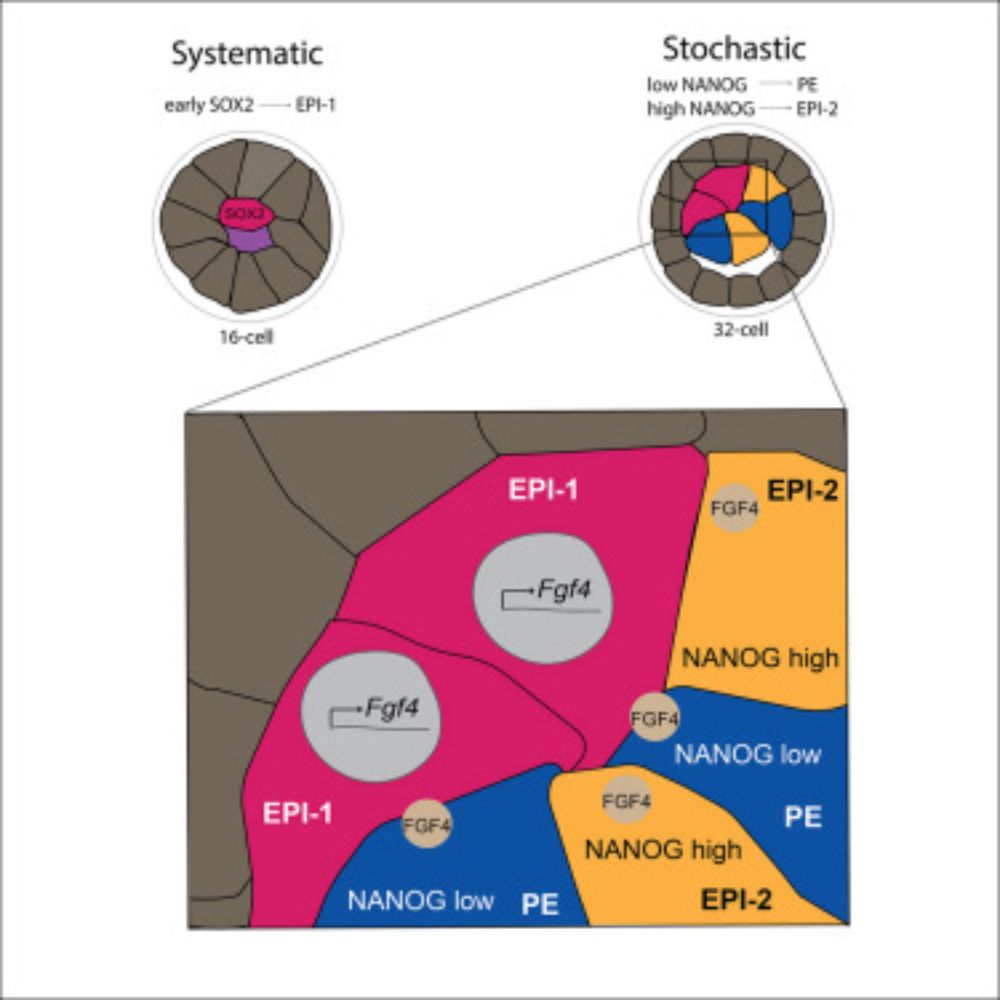
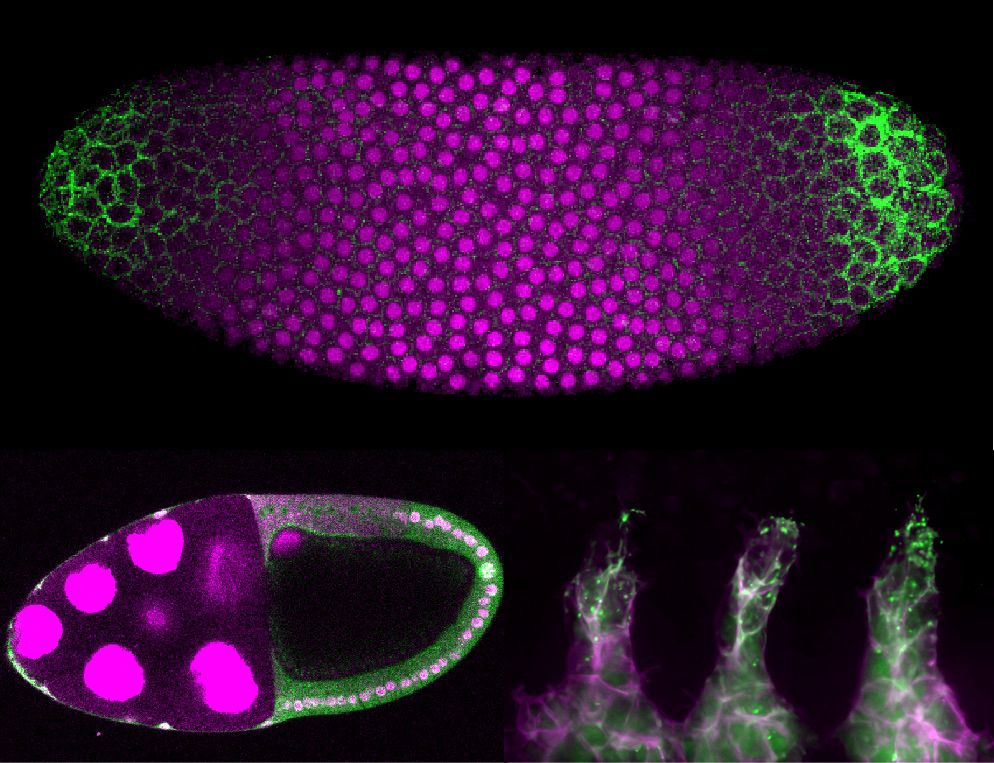
Live imaging endogenous transcription factor dynamics reveals mechanisms of epiblast and primitive endoderm fate segregation
Kim-Yip, Denberg, et al. visualize epiblast and primitive endoderm specification by
live imaging key transcription factors. They reveal systematic and stochastic features
by identifying a primary epib...
www.cell.com
Eszter Posfai
@eposfai.bsky.social
· Aug 2
Eszter Posfai
@eposfai.bsky.social
· Aug 2
Eszter Posfai
@eposfai.bsky.social
· Aug 2
Eszter Posfai
@eposfai.bsky.social
· Aug 2
Reposted by Eszter Posfai
Reposted by Eszter Posfai
Reposted by Eszter Posfai
Reposted by Eszter Posfai
Devanshi Jain
@thejainlab.bsky.social
· Apr 8
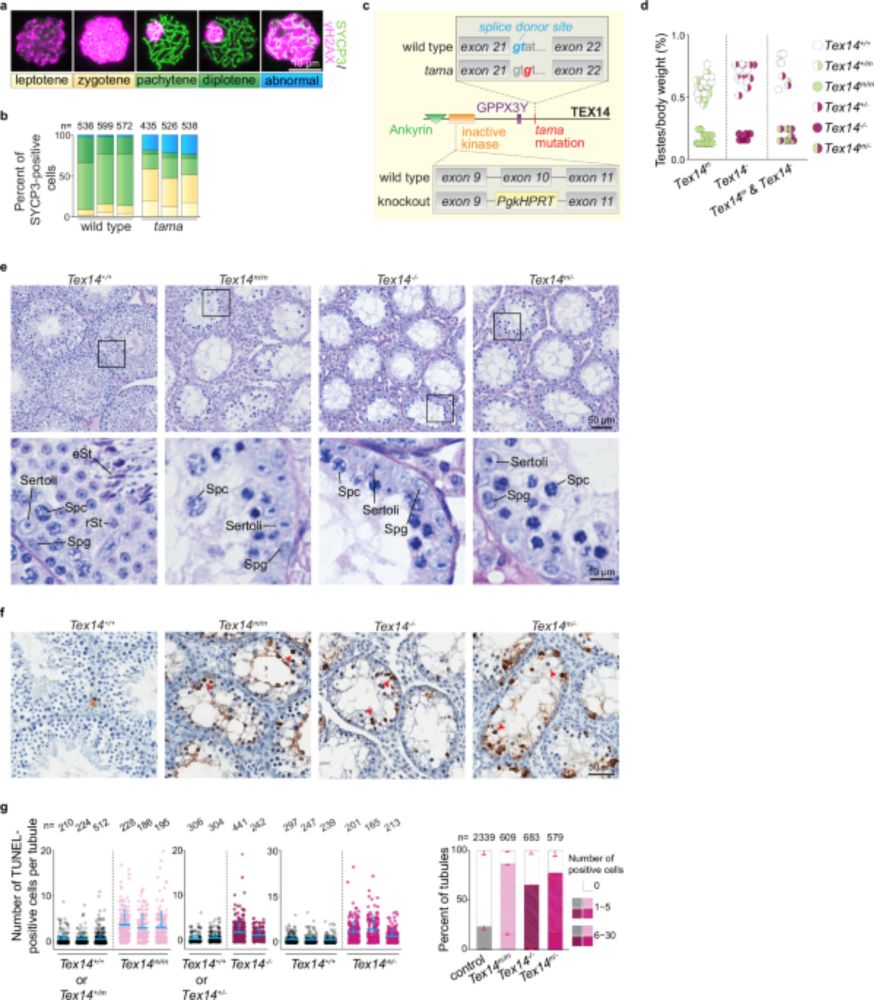
Intercellular bridges are essential for transposon repression and meiosis in the male germline - Nature Communications
A conserved feature of metazoan meiosis is that it occurs in a syncytium. Here, the authors show that intercellular bridges that connect germ cells in a syncytium are critical for ensuring proper meio...
www.nature.com
Eszter Posfai
@eposfai.bsky.social
· Mar 22
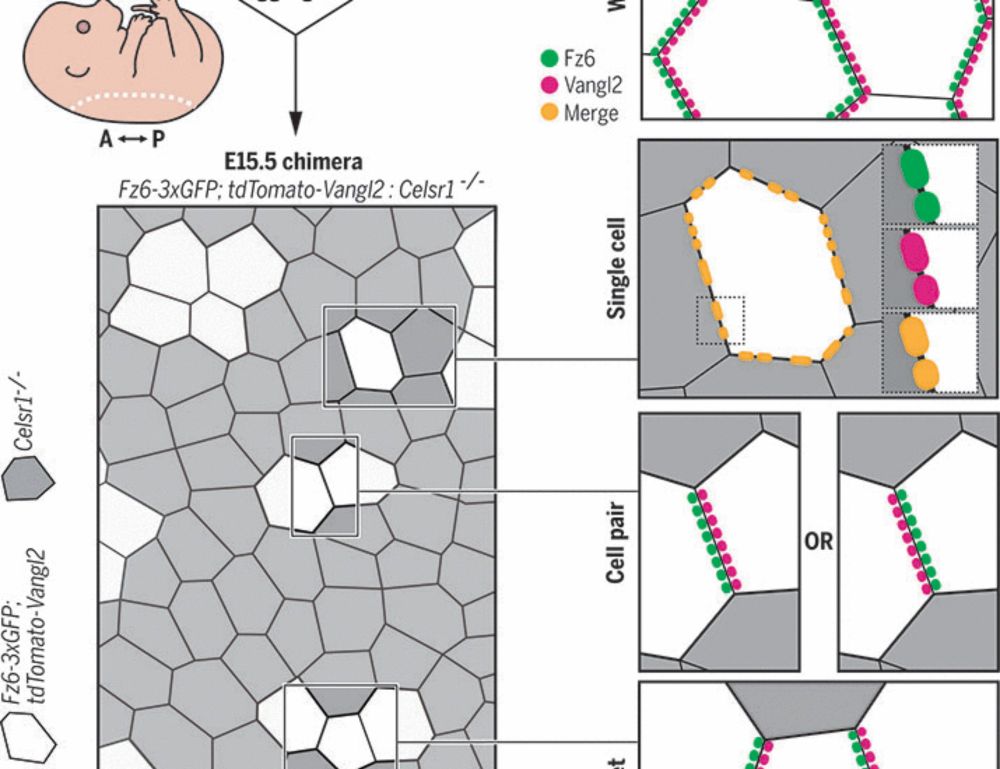
Epithelial polarization by the planar cell polarity complex is exclusively non–cell autonomous
For cells to polarize collectively along a tissue plane, asymmetrically localized planar cell polarity (PCP) complexes must form intercellular contacts between neighboring cells. Yet, it is unknown wh...
www.science.org
Reposted by Eszter Posfai
Shinuo Weng
@shinuoweng.bsky.social
· Mar 21
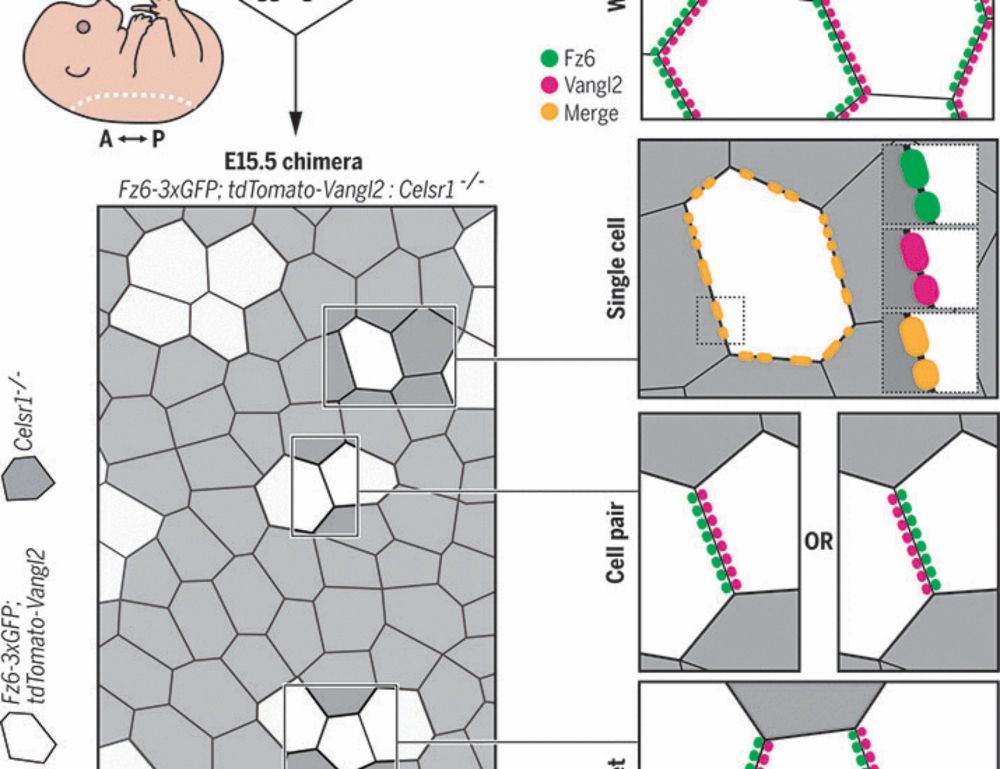
Epithelial polarization by the planar cell polarity complex is exclusively non–cell autonomous
For cells to polarize collectively along a tissue plane, asymmetrically localized planar cell polarity (PCP) complexes must form intercellular contacts between neighboring cells. Yet, it is unknown wh...
www.science.org
Eszter Posfai
@eposfai.bsky.social
· Mar 5

Generative model for the first cell fate bifurcation in mammalian development
The first cell fate bifurcation in mammalian development directs cells toward either the trophectoderm (TE) or inner cell mass (ICM) compartments in preimplantation embryos. This decision is regulated by the subcellular localization of a transcriptional co-activator YAP and takes place over several progressively asyn-chronous cleavage divisions. As a result of this asynchrony and variable arrangement of blastomeres, reconstructing the dynamics of the TE/ICM cell specification from fixed embryos is extremely challenging. To address this, we developed a live imaging approach and applied it to measure pairwise dynamics of nuclear YAP and its direct target genes, CDX2 and SOX2, key transcription factors of TE and ICM, respectively. Using these datasets, we constructed a generative model of the first cell fate bifurcation, which reveals the time-dependent statistics of the TE and ICM cell allocation. In addition to making testable predictions for the joint dynamics of the full YAP/CDX2/SOX2 motif, the model revealed the stochastic nature of the induction timing of the key cell fate determinants and identified the features of YAP dynamics that are necessary or sufficient for this induction. Notably, temporal heterogeneity was particularly prominent for SOX2 expression among ICM cells. As heterogeneities within the ICM have been linked to the initiation of the second cell fate decision in the embryo, understanding the origins of this variability is of key significance. The presented approach reveals the dynamics of the first cell fate choice and lays the groundwork for dissecting the next cell fate bifurcations in mouse development. ### Competing Interest Statement The authors have declared no competing interest.
www.biorxiv.org
Eszter Posfai
@eposfai.bsky.social
· Mar 5

Geometric, cell cycle and maternal-to-zygotic transition-associated YAP dynamics during preimplantation embryo development
During the first cell fate decision in mammalian embryos the inner cell mass cells, which will give rise to the embryo proper and other extraembryonic tissues, segregate from the trophectoderm cells, the precursors of the placenta. Cell fate segregation proceeds in a gradual manner encompassing two rounds of cell division, as well as cell positional and morphological changes. While it is known that the activity of the Hippo signaling pathway and the subcellular localization of its downstream effector YAP dictate lineage specific gene expression, the response of YAP to these dynamic cellular changes remains incompletely understood. Here we address these questions by quantitative live imaging of endogenously tagged YAP while simultaneously monitoring geometric cellular features and cell cycle progression throughout cell fate segregation. We apply a probabilistic model to our dynamic data, providing a quantitative characterization of the mutual effects of YAP and cellular relative exposed area, which has previously been shown to correlate with subcellular YAP localization in fixed samples. Additionally, we study how nuclear YAP levels are influenced by other factors, such as the decreasing pool of maternally provided YAP that is partitioned to daughter cells through cleavage divisions, cell cycle-associated nuclear volume changes, and a delay after divisions in adjusting YAP levels to new cell positions. Interestingly, we find that establishing low nuclear YAP levels required for the inner cell mass fate is largely achieved by passive cell cycle-associated mechanisms. Moreover, contrary to expectations, we find that mechanical perturbations that result in cell shape changes do not influence YAP localization in the embryo. Together our work identifies how various inputs are integrated over a dynamic developmental time course to shape the levels of a key molecular determinant of the first cell fate choice. ### Competing Interest Statement The authors have declared no competing interest.
www.biorxiv.org
Eszter Posfai
@eposfai.bsky.social
· Feb 12
Reposted by Eszter Posfai
Reposted by Eszter Posfai
Reposted by Eszter Posfai
James Briscoe
@jamesbriscoe.bsky.social
· Nov 29

Sensing of extracellular L-Proline availability by the integrated stress response determines the outcome of cell competition.
Cell competition is a quality control acting from development to the adult that eliminates cells that are less-fit than their neighbours. How winner cells induce the elimination of losers during this ...
www.biorxiv.org
Reposted by Eszter Posfai
Eszter Posfai
@eposfai.bsky.social
· Nov 21