Andrew Newman
@epinewman.bsky.social
49 followers
110 following
33 posts
Investigating the Neuronal Epigenome in Development, Evolution, & Aging.
Junior Group Leader @ Charité Universitätsmedizin Berlin
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Andrew Newman
Reposted by Andrew Newman
Reposted by Andrew Newman
Reposted by Andrew Newman
Andrew Newman
@epinewman.bsky.social
· Sep 1

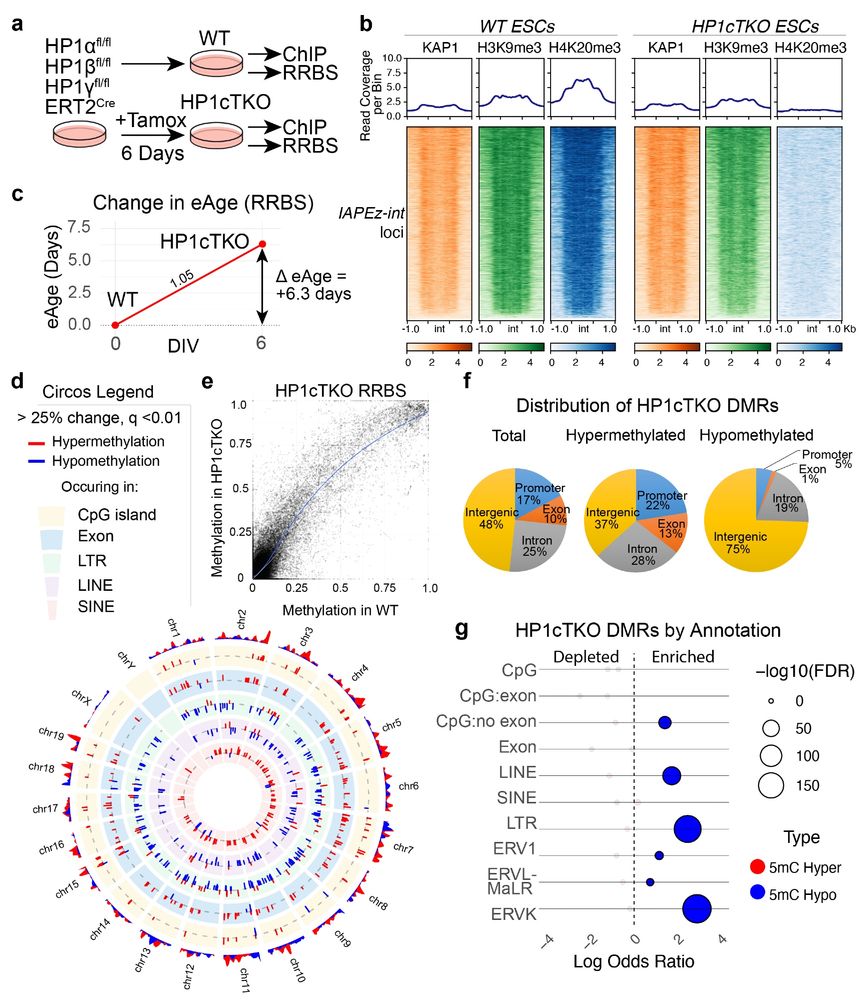
Glial reactivity and cognitive decline follow chronic heterochromatin loss in neurons - Nature Communications
Heterochromatin loss has been linked to aging and neurodegeneration. Here, the authors show that combined loss of HP1β and HP1γ in neurons results in de-repressesion of endogenous retroviruses, i...
www.nature.com
Andrew Newman
@epinewman.bsky.social
· Sep 1
Andrew Newman
@epinewman.bsky.social
· Sep 1