Daniel Friedrich
@friedrichlab.bsky.social
54 followers
71 following
18 posts
Research Group Leader @chemunicologne.bsky.social at University of Cologne (@unicologne.bsky.social) in Biomolecular #NMR Spectroscopy | We are interested in structural analysis of proteins, peptides and nucleic acids.
https://nmr.chemie.uni-koeln.de/
Posts
Media
Videos
Starter Packs
Reposted by Daniel Friedrich
BWJones
@bwjones.bsky.social
· 14d

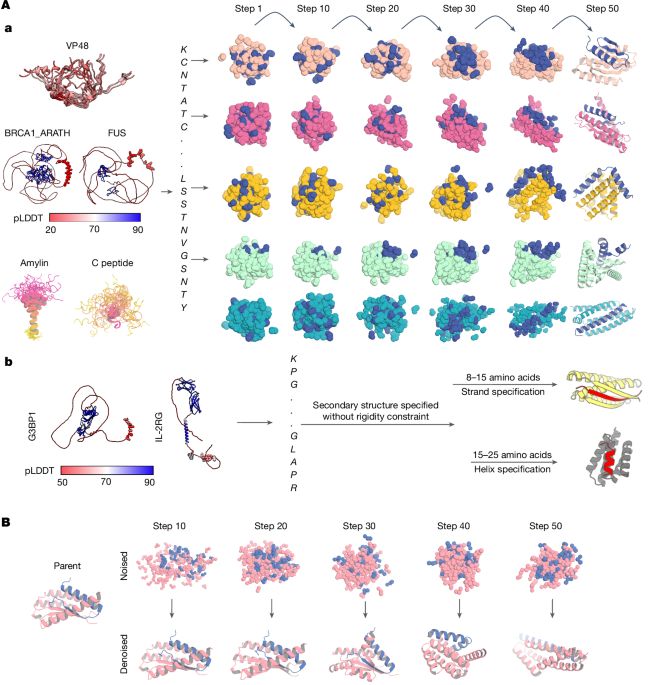
SimpleFold: Folding Proteins is Simpler than You Think
Protein folding models have achieved groundbreaking results typically via a combination of integrating domain knowledge into the architectural blocks and training pipelines. Nonetheless, given the suc...
arxiv.org
Reposted by Daniel Friedrich
Reposted by Daniel Friedrich
Reposted by Daniel Friedrich
Sigrid Milles
@millessigrid.bsky.social
· Aug 21

Promiscuous and multivalent interactions between Eps15 and partner protein Dab2 generate a complex interaction network - Nature Communications
Eps15, a protein involved in endocytosis initiation, uses small folded EH domains to bind disordered partner proteins and its own disordered tail, thus forming a dynamic network via phase separation t...
www.nature.com
Reposted by Daniel Friedrich
Team Thomma
@teamthomma.bsky.social
· Aug 15
Fantin Mesny
@mesny.bsky.social
· Aug 15

Plant-associated fungi co-opt ancient antimicrobials for host manipulation
Evolutionary histories of effector proteins secreted by fungal pathogens to mediate plant colonization remain largely elusive. While most functionally characterized effectors modulate plant immunity, ...
www.biorxiv.org
Reposted by Daniel Friedrich