Julie A. K. McDonald
@julieakmcdonald.bsky.social
170 followers
150 following
25 posts
Associate Professor at the CBRB at Imperial College London. Studying how gut microbiota-mediated colonisation resistance protects the host against infections. https://www.imperial.ac.uk/people/julie.mcdonald
Posts
Media
Videos
Starter Packs
Reposted by Julie A. K. McDonald
Reposted by Julie A. K. McDonald
Reposted by Julie A. K. McDonald
Reposted by Julie A. K. McDonald
Reposted by Julie A. K. McDonald
Reposted by Julie A. K. McDonald
Gabriele Pollara
@gpollara.bsky.social
· Jul 11
Reposted by Julie A. K. McDonald
ᐯIᑕTOᖇ ᑎIᘔET, ᗰᗪ
@nizet.bsky.social
· Jul 11
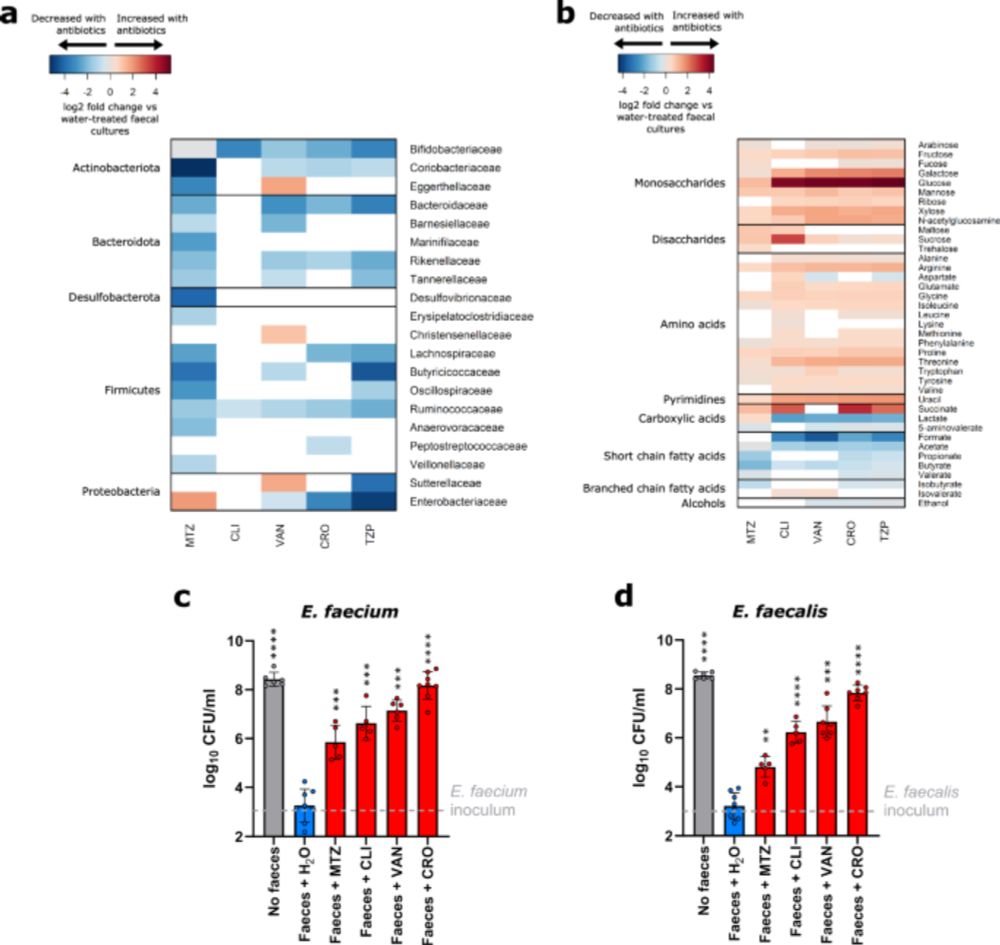
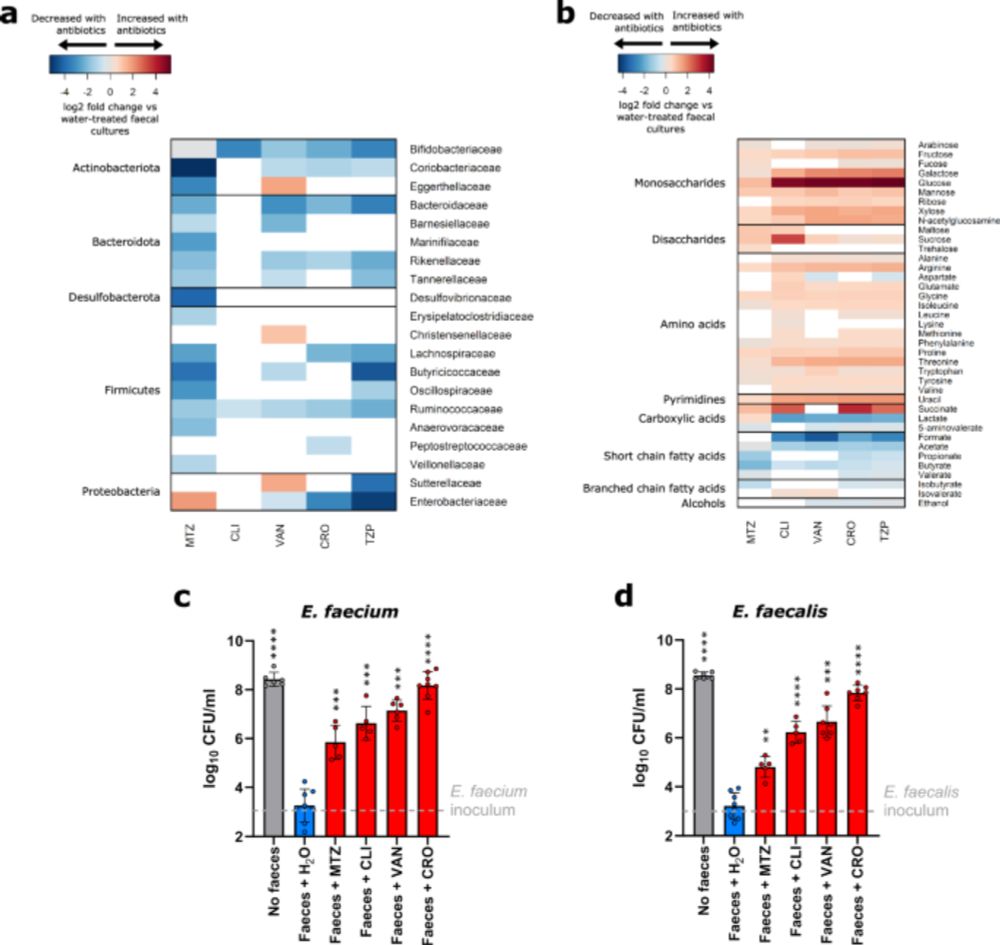
Vancomycin-resistant enterococci utilise antibiotic-enriched nutrients for intestinal colonisation - Nature Communications
Here, the authors show that vancomycin-resistant enterococci grow in the antibiotic-treated gut microbiome by utilising enriched nutrients in the presence of reduced concentrations of inhibitory micro...
www.nature.com
Reposted by Julie A. K. McDonald