Maria Dilia Palumbieri
@mariapalumbieri.bsky.social
46 followers
64 following
5 posts
Passionate about science. Interested in DNA repair and ADP ribosylation
Postdoc at the
MaxPlanck for biology of ageing
@maticlab.bsky.social
Posts
Media
Videos
Starter Packs
Pinned
The Matić Lab
@maticlab.bsky.social
· Jul 9
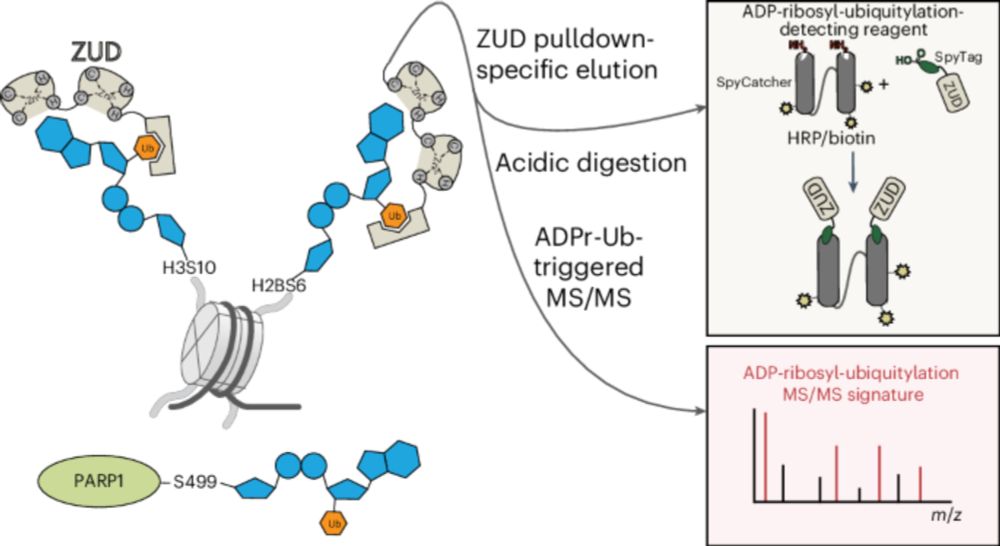
Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by Maria Dilia Palumbieri
Andrii Gorelik
@andriigorelik.bsky.social
· Jul 10

Identification of RNF114 as ADPr-Ub reader through non-hydrolysable ubiquitinated ADP-ribose - Nature Communications
Deltex E3s modify ADP-ribosylated targets with ubiquitin, creating a hybrid modification whose readers remains unknown. Here, the authors synthesise a non-hydrolysable probe that mimics the modificati...
www.nature.com
The Matić Lab
@maticlab.bsky.social
· Jul 9

Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by Maria Dilia Palumbieri
The Matić Lab
@maticlab.bsky.social
· Jul 9

Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by Maria Dilia Palumbieri
Reposted by Maria Dilia Palumbieri
Reposted by Maria Dilia Palumbieri
Reposted by Maria Dilia Palumbieri
Reposted by Maria Dilia Palumbieri








