The Matić Lab
@maticlab.bsky.social
92 followers
76 following
15 posts
We are interested in ADP-ribosylation, PARP1 signalling, proteomics, histone PTMs, protein engineering, the DNA damage response and aging
Max Planck Institute for Biology of Ageing, Cologne, Germany
Posts
Media
Videos
Starter Packs
Pinned
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 9
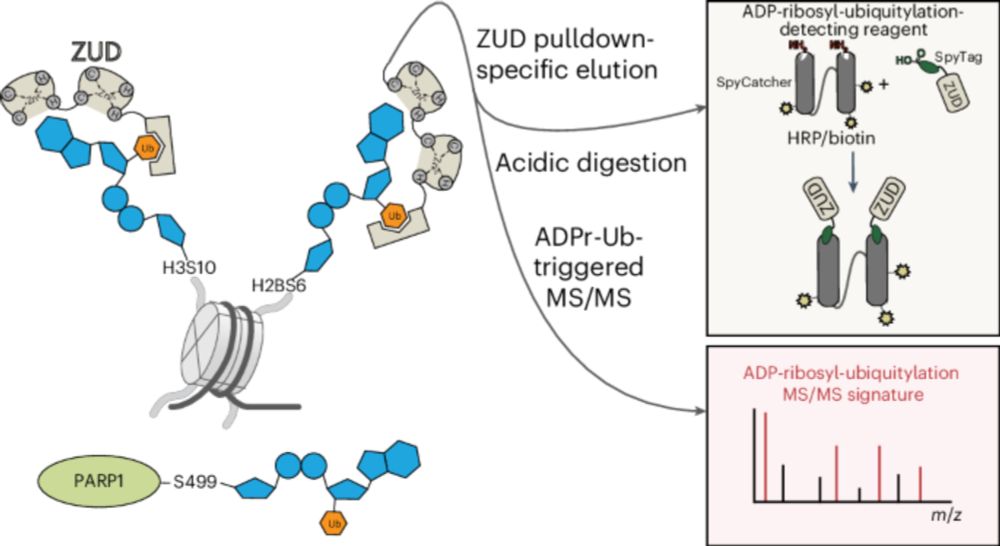
Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by The Matić Lab
Reposted by The Matić Lab
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 11
The Matić Lab
@maticlab.bsky.social
· Jul 9

Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by The Matić Lab
The Matić Lab
@maticlab.bsky.social
· Jul 9

Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by The Matić Lab
The Matić Lab
@maticlab.bsky.social
· Jul 9

Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
The Matić Lab
@maticlab.bsky.social
· Jul 9

Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation - Nature Chemical Biology
RNF114 is an E3 ligase that can recognize ADP-ribose (ADPr) and ubiquitin with separate domains. Using these domains, Kolvenbach and Palumbieri et al. developed a proteomics approach to map ADP-ribosy...
www.nature.com
Reposted by The Matić Lab
Reposted by The Matić Lab
Anthony Leung
@leunglab.bsky.social
· Mar 13

dELTA-MS: A Mass Spectrometry-Based Proteomics Approach for Identifying ADP-Ribosylation Sites and Forms
ADP-ribosylation, characterized by the addition of adenosine diphosphate ribose, can occur in both monomeric (MARylation) and polymeric (PARylation) forms. Little is known about the specific contribut...
pubs.acs.org
Reposted by The Matić Lab
Mike Cohen
@michaelnadbio.bsky.social
· Feb 25

Ubiquitin is directly linked via an ester to protein-conjugated mono-ADP-ribose | The EMBO Journal
imageimageCertain E3 ligases have been found to ubiquitylate hydroxyl groups on free NAD+ and
ADP-ribose in vitro, but the in vivo occurrence of this dual post-translational modification
has remained ...
www.embopress.org
The Matić Lab
@maticlab.bsky.social
· Feb 9
The Matić Lab
@maticlab.bsky.social
· Feb 9
The Matić Lab
@maticlab.bsky.social
· Feb 9
The Matić Lab
@maticlab.bsky.social
· Feb 9







