@thexavierlab.bsky.social
130 followers
25 following
27 posts
Genes to functions. Microbiome in health & disease. Computational biology. Chemical biology. Innate & adaptive immunity.
http://broadinstitute.org/xavier-lab
https://molbio.massgeneral.org/faculty/343
Posts
Media
Videos
Starter Packs
Reposted
Reposted
Reposted
Kuchroo Lab
@kuchroolab.bsky.social
· May 23
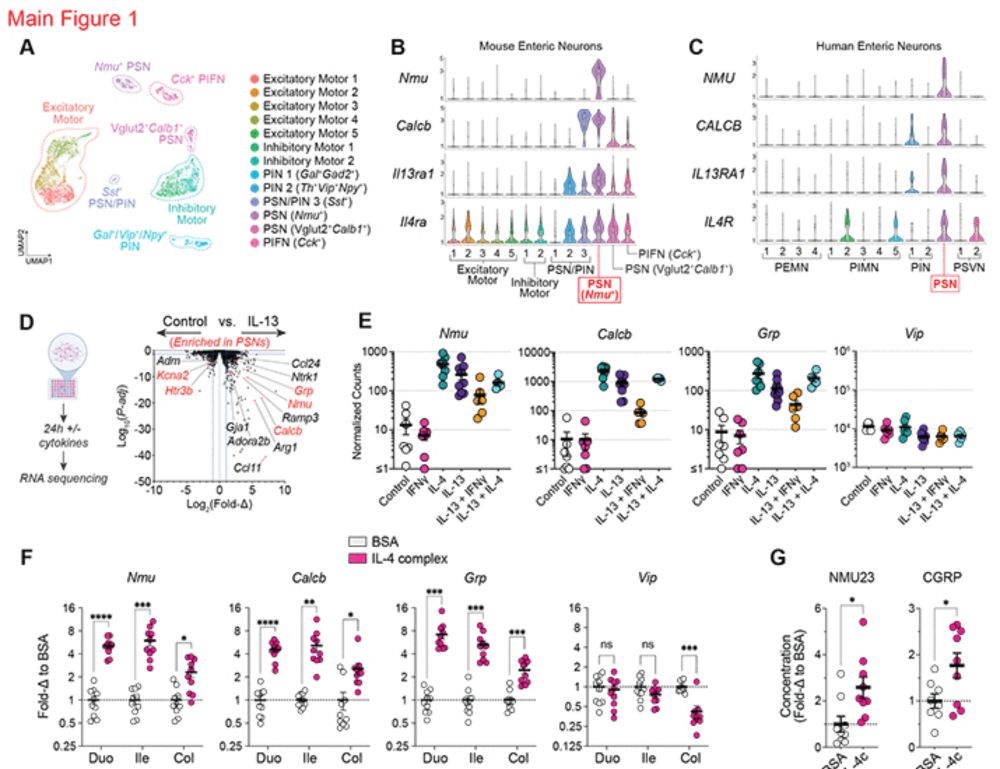
Type 2 cytokines act on enteric sensory neurons to regulate neuropeptide-driven host defense
Enteric nervous system (ENS)–derived neuropeptides modulate immune cell function, yet our understanding of how inflammatory cues directly influence enteric neuron responses during infection is conside...
www.science.org