Tom Cumming
@tomcumming.bsky.social
38 followers
43 following
20 posts
PhD student at Institut Pasteur studying commitment to cell death downstream of the caspases in epithelia.
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Tom Cumming
Aline Grata
@alinegrata.bsky.social
· Jul 4
preLights
@prelights.bsky.social
· Jul 4
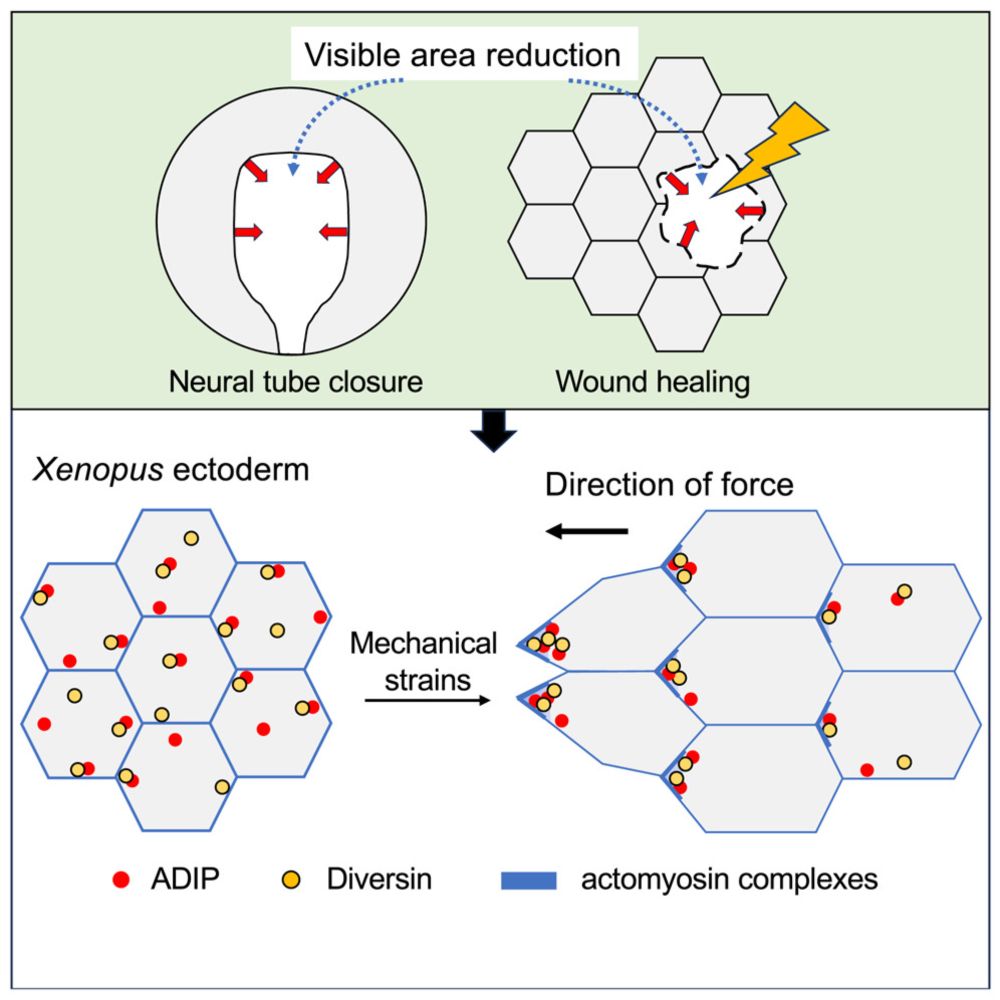
Mechanical cues organize planar cell polarity during vertebrate morphogenesis and embryonic wound repair - preLights
Stretch it, wound it, pull it: ADIP-Diversin polarizes anyway. Characterization of a new mechanosensitive polarity module.
prelights.biologists.com
Tom Cumming
@tomcumming.bsky.social
· May 21
Tom Cumming
@tomcumming.bsky.social
· May 21
Tom Cumming
@tomcumming.bsky.social
· May 21
Tom Cumming
@tomcumming.bsky.social
· May 21
Tom Cumming
@tomcumming.bsky.social
· May 21
Tom Cumming
@tomcumming.bsky.social
· May 21
Tom Cumming
@tomcumming.bsky.social
· May 21
Reposted by Tom Cumming
Arthur Michaut 🔬🐣
@amichaut.bsky.social
· May 12

Direct measurements of active forces and material properties unveil the active mechanics of early embryogenesis
Despite progress in probing tissue mechanics, direct long-term measurements in live embryonic epithelia are lacking. This limits our understanding of amniote embryonic morphogenesis, which takes place...
www.biorxiv.org
Reposted by Tom Cumming