https://updates.chemo.org.uk/CDF_Forms/SELIN1.html
🚨 Treatment criterion (#5 and 13) updated; T...
https://updates.chemo.org.uk/CDF_Forms/SELIN1.html
🚨 Treatment criterion (#5 and 13) updated; T...
The Malaysian National Pharmaceutical Regulatory Agency has approved an sNDA for selinexor for the treatment of adult patients with R/R DLBCL who have had ≥2 prior lines of therapy and are ineligible for auto-HSCT.
Read more: https://loom.ly/GDZmYEI
#Lymphoma #lymsm #MedNews

The Malaysian National Pharmaceutical Regulatory Agency has approved an sNDA for selinexor for the treatment of adult patients with R/R DLBCL who have had ≥2 prior lines of therapy and are ineligible for auto-HSCT.
Read more: https://loom.ly/GDZmYEI
#Lymphoma #lymsm #MedNews



Hong Kong Special Administrative Region approves supplemental new drug application for selinexor, an XPO1 inhibitor, for adult patients with R/R DLBCL-NOS after ≥2 lines of systemic therapy who are ineligible for HSCT.
Read more: https://loom.ly/1YZkZpU
#lymphoma #lymsm #MedNews

Hong Kong Special Administrative Region approves supplemental new drug application for selinexor, an XPO1 inhibitor, for adult patients with R/R DLBCL-NOS after ≥2 lines of systemic therapy who are ineligible for HSCT.
Read more: https://loom.ly/1YZkZpU
#lymphoma #lymsm #MedNews
www.nature.com/articles/s41...

www.nature.com/articles/s41...
#Hemato2025 @sehhematologia.bsky.social

#Hemato2025 @sehhematologia.bsky.social

link.springer.com/article/10.1...

link.springer.com/article/10.1...

Sosana Delimpasi shared the findings at #EHA2025:
👉 buff.ly/H97ScVm
#MMSM #Myeloma #CTSM #HemOnc #BloodSky #HemeSky

Sosana Delimpasi shared the findings at #EHA2025:
👉 buff.ly/H97ScVm
#MMSM #Myeloma #CTSM #HemOnc #BloodSky #HemeSky

We hit them with importazole (import blocker) and selinexor (export inhibitor).
🔥 Targeting nuclear import decreases the hybrid cell pool and enhances chemoradiation sensitivity, opening a new therapeutic avenue against GBM. (5/9)
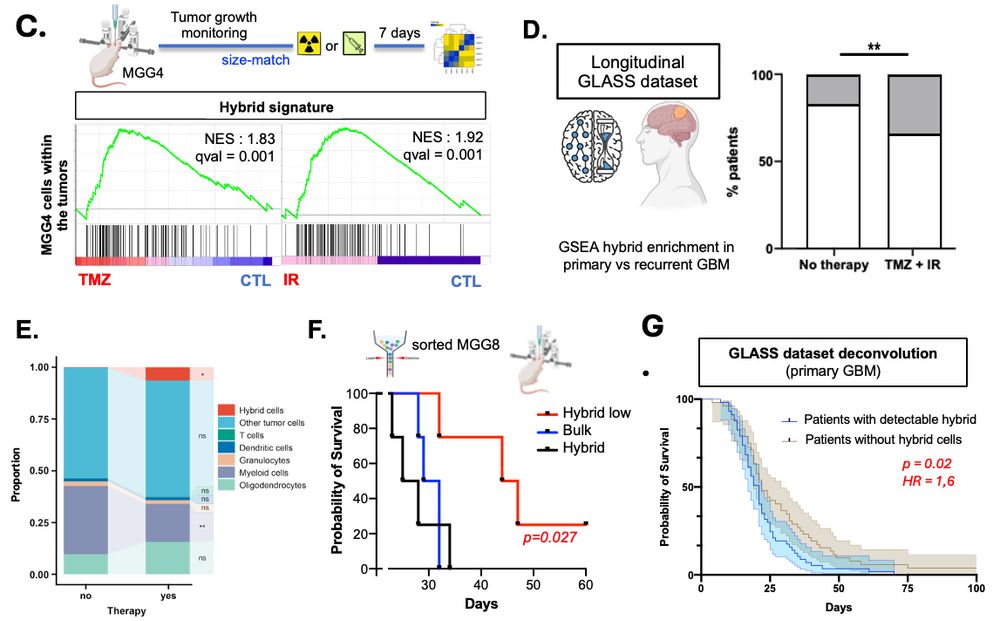
We hit them with importazole (import blocker) and selinexor (export inhibitor).
🔥 Targeting nuclear import decreases the hybrid cell pool and enhances chemoradiation sensitivity, opening a new therapeutic avenue against GBM. (5/9)



Watch here ➡️ ow.ly/aTXv50WhTZx
#ASCO25 #GynOnc #Gyncsm #EndometrialCancer

Watch here ➡️ ow.ly/aTXv50WhTZx
#ASCO25 #GynOnc #Gyncsm #EndometrialCancer

