Reposted by: Andrew Newman
I wrote about this problem in The Death of Expertise. (My solution, despite years of good evals, was to have other faculty observe my class and give me feedback, even when I was senior and tenured.)
by @rosehorowitch.bsky.social
www.theatlantic.com/ideas/archiv...
by @rosehorowitch.bsky.social
www.theatlantic.com/ideas/archiv...

Reposted by: Andrew Newman
One week left to register for the 1st conference on assembloids at @cshlnews.bsky.social this December. Spectacular list of speakers (see below) tackling cell-cell interactions across tissues and organs
Two amazing keynotes:
Ruslan M. Medzhitov & Joanna Wysocka
#assembloids #organoids
Two amazing keynotes:
Ruslan M. Medzhitov & Joanna Wysocka
#assembloids #organoids

Reposted by: Andrew Newman
scAgeClock: a single-cell transcriptome based human aging clock model using gated multi-head attention neural networks https://www.biorxiv.org/content/10.1101/2025.08.29.673183v1
This has been a long effort, with many contributors from
Charité Berlin and a wonderful collaboration with researchers from RIKEN IMS.
full paper here:
www.nature.com/articles/s41...
Charité Berlin and a wonderful collaboration with researchers from RIKEN IMS.
full paper here:
www.nature.com/articles/s41...

Glial reactivity and cognitive decline follow chronic heterochromatin loss in neurons - Nature Communications
Heterochromatin loss has been linked to aging and neurodegeneration. Here, the authors show that combined loss of HP1β and HP1γ in neurons results in de-repressesion of endogenous retroviruses, i...
www.nature.com
Our study provides an invaluable insight into how heterochromatin loss with concomitant (re)activation of ERVs can drive core components of neurodegeneration by stimulation of immune pathways and the integrated stress response.

...as well as age-dependent deficits in paired-pulse inhibition and spatial memory, measured here in the barnes maze.

Alongside this chronic inflammatory state we observed age-dependent reductions in dendritic complexity in the hippocampus...

However, in the chronic environment of the brain, the prolonged production of complement C3 coincided with increased number of microglia as well as increased microglial activation as measured by CD68 subcellular compartments.

While we could not observe direct affects to complement in this acute paradigm, we could observe specific effects to CCL5 (RANTES) production and IRF7 phosphorylation and nuclear localization.

We attempted to investigate this in an in vitro system, where we applied IAP ssRNA to mixed glial cultures, alongside pseudo-uracil (ψ) and scramble controls.

At the protein level, this is even more dramatic, where you can observe the accumulation of C3 protein in the astrocytes, and specifically their endfeet.

We were shocked to find that in the IAP ERV-rich hippocampus, Complement C3 RNA production could be seen in foci we could attribute to Slc1a3 astrocytes.

So HP1β & HP1γ seem to be essential for repeat silencing, but what is the consequence in the brain?
Our transcriptomic data suggested multiple pathways related to chronic inflammation, elevated integrated stress response, and notably, the complement cascade.
Our transcriptomic data suggested multiple pathways related to chronic inflammation, elevated integrated stress response, and notably, the complement cascade.

The TLDR is that in the DKO brain samples we could additionally observe loss of 5mC at ERVK (IAP) elements, with corresponding increases in 5hmC. Several de novo 5mC methylation also appears to take place in HP1β cortices, suggesting that HP1β is essential for the 5mC fidelity.

We then sought to corroborate this in the brain. Given the abundance of 5hmC in the brain, we performed oxidative RRBS (OvationRRBS system, RIP) to simultaneously profile 5mC and 5hmC from our mutant hippocampi.
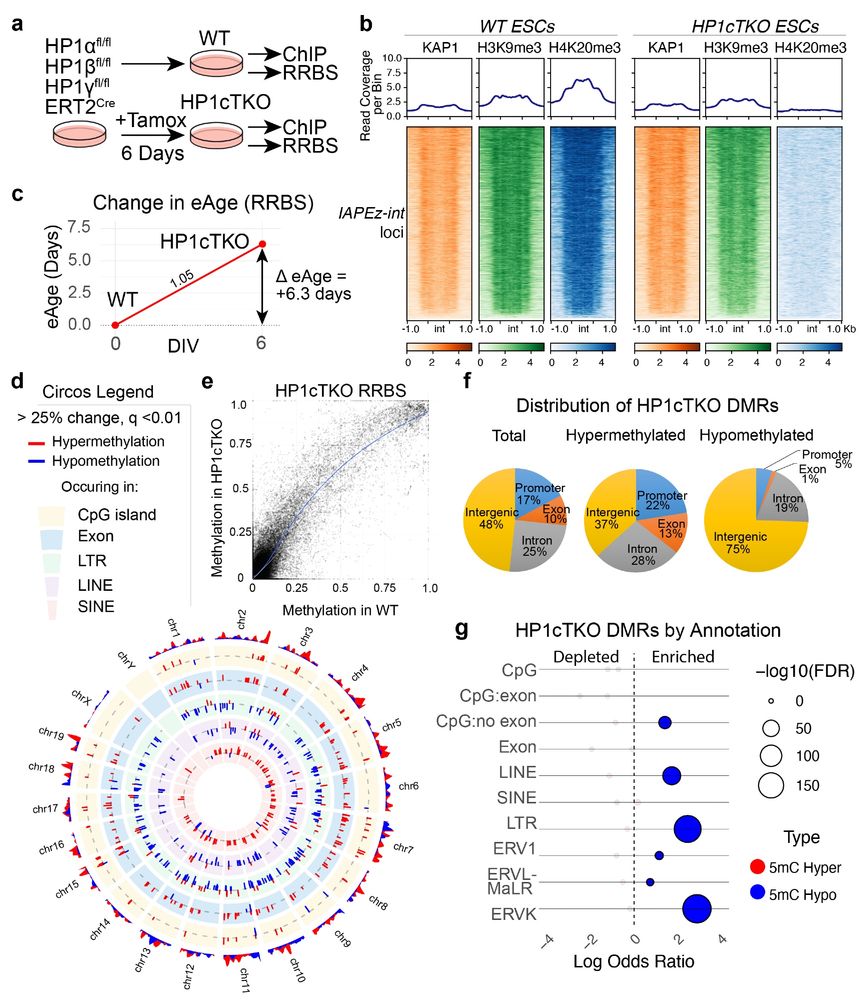
We then tested how HP1 deficiency effects DNA methylation, using an ES cell line. We could see that in stem cells triple deficient for HP1 proteins, LINE and LTR elements (including ERVK) became DNA hypomethylated.
When we re-introduced HP1γ-GFP into the DKO cortex we could recover H4K20me3 levels, albeit not to the same extent as adjacent WT interneurons.

So why was the double loss of HP1 proteins resulting in this loss of ERV silencing? It turns out that HP1γ is necessary for secondary H4K20me3 deposition.

And notably, while Emx1Cre results in HP1 proteins being deleted from neurons and astrocytes, IAPs are only de-repressed in neurons. Something you can appreciate here in the hippocampal formation:

A large proportion of what is de-repressed are IAP (Intracisternal Alpha Particle) elements, mouse specific ERVK family members, which are the closest functional analogs to human HERV-K.
With a consensus RNA probe, we can visualise locations of IAP transcripts in situ.
With a consensus RNA probe, we can visualise locations of IAP transcripts in situ.

Using bulk RNAseq, we found that deletion of these two genes in combination resulted in derepression of ERV elements (here listed as green under LTR Class) across the genome, primarily those with a smaller Kimura distances, corresponding to evolutionarily younger sequences.

We asked if we could mimic age-related heterochromatin loss by deleting two members of the HP1 family, HP1β and HP1γ, and observe the consequences in the Cortex of a living mouse using Emx1Cre.
Heterochromatin formation is dependent on Heterochromatin Protein 1 (HP1), a protein that dimerises and binds strongly to K9me3 on histone 3 (H3K9me3) initiating condensate formation and phase separation.

One way ERVs are silenced is by the KAP1 repressor complex, where the element is recognised by a KRAB-containing Zinc finger and the result is silencing via H3K9me3, H4K20me3 histone methylation and 5mC DNA methylation.
royalsocietypublishing.org/doi/full/10....
royalsocietypublishing.org/doi/full/10....

However, normally most are silenced so they don’t cause any negative effects. Misregulation of ERV silencing is known to cause several physiological problems including inflammation, cancer and potentially neurodegeneration.
(See this review for more:
nature.com/articles/s41... )
(See this review for more:
nature.com/articles/s41... )

Activation of human endogenous retroviruses and its physiological consequences - Nature Reviews Molecular Cell Biology
Human endogenous retroviruses (HERVs) are relics of ancient retroviral infections, which provide coding and non-coding sequences to the human genome. Emerging evidence reveals how HERVs contribut...
nature.com
Endogenous Retroviruses (ERVs) are relics of viruses that integrated into the genome at some point in the past. Some of these sequences can be 'exapted' to be used as regulatory sequences by the host genome.
We initiated this study based on an emerging consensus that aging cells often lose compartmentalisation in the nucleus, including the loss of H3K9me3 heterochromatin resulting in elevated transcription of repetitive elements such as endogenous retroviruses (ERVs).

Our study on chronic de-repression of ERVs in the brain is finally out in
@natcomms.nature.com
We dove deep into the molecular mechanisms controlling their repression as well as the physiological consequences of their extended expression in the cortex over the lifespan of a mouse.
a 🧵 👇
@natcomms.nature.com
We dove deep into the molecular mechanisms controlling their repression as well as the physiological consequences of their extended expression in the cortex over the lifespan of a mouse.
a 🧵 👇

Reposted by: Andrew Newman
Happy to have this published …https://www.science.org/doi/10.1126/sciadv.adr6698
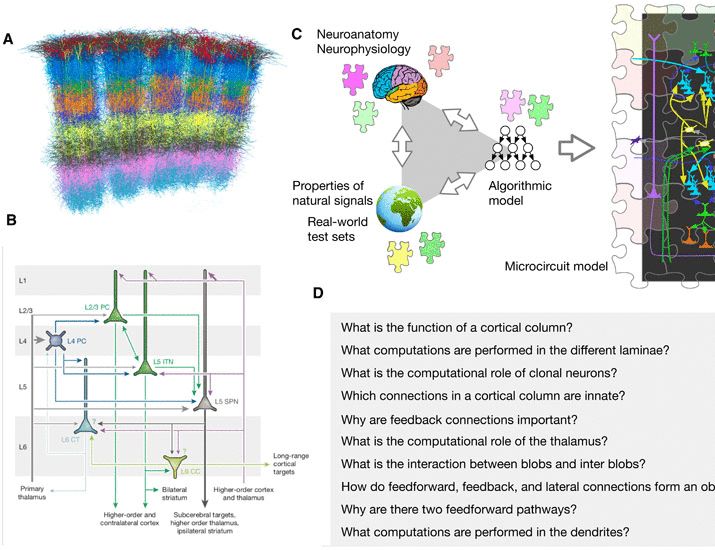
A detailed theory of thalamic and cortical microcircuits for predictive visual inference
A generative vision model offers a detailed theory for cortical columns, thalamus, and computations in cortical circuits.
www.science.org
